States of Matter: Solids, Liquids, and Gases
Matter is anything that occupies space and has mass, consisting of tiny particles like atoms and molecules. Solids have closely packed particles, liquids have less densely packed particles that can flow, and gases have spread out particles. Solids retain their shape, liquids take the shape of their
7 views • 11 slides
Kinetic Theory of Gases: Key Concepts and Equations
Exploring the kinetic theory of gases, this content covers essential concepts such as ideal gas behavior, molar mass, the equation of state, and isobaric/isothermal processes. Discover the relationship between pressure, volume, and temperature in gases, along with practical examples and calculations
0 views • 49 slides
Gas Laws and Properties: Understanding the Behavior of Gases
Gas laws govern the behavior of gases and their properties in various conditions. From the total pressure of gas mixtures to calculating partial pressures, understanding the relationship between pressure, volume, temperature, and amount of gas is crucial. Effusion and diffusion play key roles in how
1 views • 16 slides
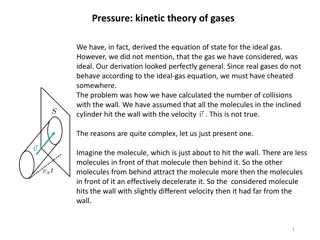
Insight into Kinetic Theory of Gases and Maxwell Velocity Distribution
The discussion delves into the kinetic theory of gases, highlighting the deviations from ideal gas behavior and the derivation of the Maxwell velocity distribution. It explores the intricacies of molecule-wall collisions, Maxwell's assumptions, the Gaussian distribution, and the concept of reversibl
0 views • 8 slides
The Noble Gases: Properties, Sources, and Uses
The noble gases, including helium, neon, argon, krypton, xenon, radon, and Oganesson, are characterized by their low reactivity due to a complete electron configuration. They have diverse applications, such as forming inert atmospheres, medical treatments, and lighting technologies. Naturally occurr
0 views • 16 slides
Kinetic Molecular Theory and States of Matter in Physical Pharmacy
The lecture by Assistant Prof. Dr. Fouadalssady in physical pharmacy delves into the Kinetic Molecular Theory, elucidating how gases consist of particles in constant motion with negligible volume. It explains the relationship between kinetic energy, temperature, and the transition from gas to liquid
0 views • 10 slides
Gases and Plasmas in the Atmosphere
Delve into the concepts of gases and plasmas in the atmosphere, exploring topics such as fluid pressure, buoyancy, and the unique properties of gases compared to liquids. Discover how the balance between kinetic energy and gravity determines the thickness of our atmosphere and why it is essential fo
0 views • 25 slides
Exploring the States of Matter Through Kinetic Molecular Theory
Delve into the fascinating world of matter states with the Kinetic Molecular Theory. Learn about the properties and behaviors of solids, liquids, gases, and plasma. Understand how particle movement, energy, and spacing differ across these states, shaping their unique characteristics.
1 views • 10 slides
Kinetic Theory of Gases and Thermodynamics
Explore the fundamental principles of the kinetic theory of gases, including the five postulates and the relationship between macroscopic properties and microscopic phenomena. Delve into the concept of entropy and thermodynamics, along with the behavior of ideal and real gases. Gain insights into th
0 views • 116 slides
Kinetic and Potential Energy: A Visual Exploration
Delve into the concepts of kinetic and potential energy through engaging visuals and explanatory content. Learn about the factors affecting kinetic energy, compare energy levels between objects in motion, explore the calculation of kinetic energy, and discover the storage and examples of potential e
0 views • 15 slides
States of Matter and Kinetic Theory
Matter is anything that occupies space and has mass, existing in solid, liquid, gas, and plasma states. The states of matter depend on the arrangement and motion of atoms. Solids have fixed shapes, liquids take the shape of their container, and gases fill the volume of their container. The Kinetic T
1 views • 18 slides
States of Matter: Kinetic Molecular Theory and Phase Transitions
Exploring the fundamental concepts of the states of matter, this content delves into the Kinetic Molecular Theory, explaining how matter changes from solid to liquid to gas based on particle movement and energy levels. It further discusses the nature of gases and liquids, vaporization, evaporation,
1 views • 17 slides
Matter: Solids, Liquids, Gases, and Fluids
Matter exists in various states - solid, liquid, gas, and fluid. Solids have atoms closely packed, liquids have more freedom but still cohesion, gases have atoms spread out, and fluids flow like liquids or gases. Mass density characterizes matter based on atom proximity. Gas pressure results from mo
1 views • 22 slides
Fascinating World of Gases and Plasmas in Chapter 14
Delve into the intriguing realm of gases and plasmas as we transition from liquids in Chapter 13. Understand the dynamics of fluid pressure, buoyancy, and the unique characteristics that differentiate gases from liquids. Explore examples illustrating the concepts and ponder over the mysteries of our
0 views • 28 slides
Kinetic Energy and Work in Physics
Kinetic energy is the energy possessed by moving objects, allowing them to do work. When a force acts on an object causing it to displace, work is done, and the object's kinetic energy changes. The work-energy theorem states that work done on an object equals the change in its kinetic energy. Real-w
1 views • 9 slides
Kinetic Theory of Matter and Phases
Explore the fundamental concepts of the Kinetic Theory of Matter, including the three pillars of kinetic energy and forces of attraction, which determine the states of matter like solid, liquid, gas. Learn about temperature, phase changes, and the phases of matter, emphasizing the role of kinetic en
0 views • 17 slides
Energy: Potential and Kinetic Forms in Grade 7 Natural Sciences
Energy in various forms is explored in Grade 7 Natural Sciences, with a focus on potential and kinetic energy. Energy is the ability to do work and exists in different types like heat, chemical, electromagnetic, nuclear, and mechanical. The sun serves as a primary energy source. Potential energy is
1 views • 11 slides
Storage and Manipulation of Liquefied Gases with Dewars and Cryostats
Storage and manipulation of liquefied gases involve using specialized equipment like Dewars and Cryostats to minimize heat transfer and maintain low temperatures. Dewars, invented by James Dewar, are double-walled vacuum vessels designed to store liquefied gases with minimal losses. The use of vacuu
4 views • 11 slides
The Kinetic Theory of Gases and Ideal Gas Model
Explore the Kinetic Theory of Gases, the Molecular Model of Ideal Gases, and concepts like pressure, temperature, and heat capacity. Understand the fundamental properties of gases, including the distribution of molecular speeds and equations that describe gas behavior. Learn about the mole concept,
1 views • 23 slides
The Behavior of Gases: A Comparison of Real and Ideal Gases
This chapter delves into the behavior of gases, contrasting real gases with the ideal gas model. It explores the P-V-T relationships of gases, highlighting the differences between ideal and real gas behaviors based on molecular properties. The critical point where coexisting gas and liquid phases co
1 views • 40 slides
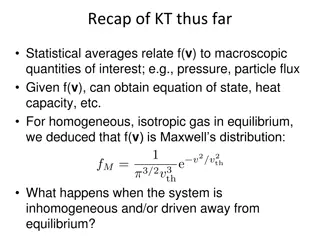
Insights into Non-equilibrium Kinetic Theory: Inhomogeneous Systems
Statistical averages in kinetic theory connect distribution functions to macroscopic properties like pressure and particle flux. When systems are inhomogeneous or away from equilibrium, local equilibrium breaks down, leading to slow relaxation processes towards global equilibrium. The evolution of p
1 views • 12 slides
Gases in the Environment and Their Impact on Earth
Gases such as CO, CO2, SO2, O2, and O3 play a crucial role in shaping our environment. This chapter delves into the properties of gases, the three states of matter, pressure exerted by gases, atmospheric pressure measurement methods, and units of pressure used in scientific fields.
0 views • 25 slides
Basics of Gas Laws and Kinetic Theory
The fundamental concepts of gas laws, the nature of gases, kinetic theory, ideal gases, gas pressure, and more. Learn about the behavior of gases, ideal gas properties, gas pressure measurements, and standard conditions.
0 views • 21 slides
Rotational Kinetic Energy
Learn about the principles of rotational kinetic energy, including translational and rotational components. Solve problems involving kinetic energy, work, and angular velocity. Explore concepts through examples and whiteboards. Understand how to calculate the total kinetic energy and work done in ro
0 views • 9 slides
Kinetic Molecular Theory
Gases consist of high-energy particles with no definite shape or volume, taking the shape of their container. The Kinetic Molecular Theory explains the behavior of gases based on particle motion, temperature, and pressure, with fundamental principles such as negligible particle volume, random motion
0 views • 20 slides
Kinetic-Molecular Theory: Non-Ideal Gases Overview
Kinetic-Molecular Theory of Gases, Boltzmann-Maxwell Distributions, and the comparison between Ideal and Non-Ideal Gases. Understand correction factors and laws like Boyle's Law, Charles's Law, and Avogadro's Law. See how the concepts of pressure, volume, temperature, and collisions shape the behavi
2 views • 19 slides
Exploring Energy: Potential vs. Kinetic Concepts
Dive into the concepts of potential and kinetic energy through labs, lectures, and discussions. Get ready for quizzes and tests on potential and kinetic energy, with opportunities for retakes. Understand the equations, types, and measurements associated with energy in motion. Explore real-world exam
0 views • 12 slides
Molecules in Air and Water Movement
Molecules move differently in air and water due to their kinetic properties. Explore how molecules cross cell membranes and the interaction between spray and air molecules. Dive into senses of taste and smell, understand the kinetic theory of gases, and observe the behavior of molecules in hot and c
0 views • 11 slides
Physical Characteristics and Laws of Gases: Kinetic Theory and Gas Properties
Explore the physical characteristics of gases, including the Kinetic Theory model, diffusion, effusion, real gases, and properties like pressure, volume, temperature, and number of moles. Discover Boyle's Law, Charles's Law, and practical gas volume calculations. Unveil the key principles of pressur
1 views • 22 slides
On-Chip Superconducting Kinetic Inductance Fourier Transform Spectrometer for mm-Astronomy
This innovative on-chip spectrometer offers a compact solution for mm-wave astronomy research, utilizing superconducting kinetic inductance technology. Learn about the motivation behind its development, the concept of kinetic inductance in superconductors, and the advancements in non-linear kinetic
0 views • 11 slides
Hazardous Gases and Compressed Cylinders Safety Guidelines
Proper handling and storage of hazardous gases in compressed cylinders are critical to prevent accidents and ensure workplace safety. Toxic, reactive, flammable, and inert gases pose various risks that must be managed effectively. This comprehensive guide covers key safety measures for storage, usag
0 views • 5 slides
Collision Scenarios and Kinetic Energy Analysis
In these scenarios, different air track gliders and objects collide head-on, resulting in their velocities and kinetic energy changing. The post-collision speeds and the lost kinetic energy are calculated for each scenario. Additionally, a bullet collides with a block of wood, leading to an evaluati
0 views • 17 slides
Understanding Gas Behavior: Boltzmann Distribution and Kinetic Energy
Explore the concepts of Boltzmann distribution, average particle speed, average kinetic energy, and internal energy of a gas. Learn how statistical mechanics describes the distribution of particle speeds in an ideal gas and the relationship between kinetic energy, temperature, and pressure. Discover
0 views • 7 slides
Chemical Characteristics of Lake: Dissolved Gases Oxygen, CO2 & More
Explore the chemical characteristics of lake waters, focusing on dissolved gases like oxygen, carbon dioxide, and others. Learn about the solubility of gases in water, factors affecting their solubility, and the importance of these gases in aquatic ecosystems.
0 views • 19 slides
Derivation of Fundamental Equation Kinetic Theory and Kinetic Molecular Theory
Explore the derivation of the fundamental equation of the kinetic theory and the kinetic-molecular theory, discussing the number of molecules in a gas, their masses, movements, collisions, impulses, and the resulting pressure in the system. Discover how kinetic energy is derived based on temperature
0 views • 33 slides
Understanding Kinetic Molecular Theory of Gases
Explore the principles of the Kinetic Molecular Theory as it relates to gases, including key characteristics, gas behavior perspectives, and the fundamental assumptions underlying the theory. Gain insights into the composition, movement, and collisions of gas particles essential for understanding ga
0 views • 20 slides
Understanding Kinetic Theory of Matter & Its Effects on States of Matter
Learn about the kinetic theory of matter, which explains the constant motion of particles in solids, liquids, and gases. Explore how temperature and pressure impact the state of matter, including changes from solid to liquid to gas. Discover the relationship between temperature, pressure, and the vo
0 views • 10 slides
Understanding Kinetic Theory of Gases and Maxwell Velocity Distribution
Explore the kinetic theory of gases, Maxwell velocity distribution, and reversible processes in gas to understand the behavior of real gases beyond the ideal gas equation. Learn about Maxwell's assumptions, Gaussian distribution, and the concept of equilibrium states in gas dynamics.
1 views • 8 slides
Understanding Gas Laws and the Kinetic Molecular Theory
Explore the fundamentals of gas laws, kinetic molecular theory, and Dalton's law in this informative content. Learn about pressure, volume, and how gases behave based on the principles of the kinetic theory. Dive into examples illustrating Dalton's law and how to calculate total pressures in gas mix
0 views • 15 slides
Kinetic Theory of Gases and Effusion Studies
Explore the key ideas in kinetic theory, including pressure calculation, internal energy density, velocity distributions, and effusion processes in classical ideal gases. Understand the validity of assumptions made and the comparison of particle speeds inside and outside a container.
1 views • 8 slides