Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Aldol Condensation Reaction: Preparation of Chalcones
Chalcones are important unsaturated aromatic ketones that serve as biogenetic precursors of flavonoids and isoflavonoids. They have various medicinal and pharmaceutical applications due to their biological activities. Chalcones are easily synthesized compounds with potential therapeutic uses, making
2 views • 13 slides
Understanding the Seliwanoff Color Reaction and its Significance
The Seliwanoff color reaction, discovered by Russian chemist Feodor Feodorovich Selivanov, is used to differentiate between aldoses and ketohexoses based on their dehydration and reaction with resorcinol in acidic conditions. Ketoses like fructose react faster than aldoses like glucose, leading to a
3 views • 20 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Understanding Energy: Types, Potential, and Kinetic
Dive into the world of energy with a comprehensive guide covering the definition, types, and characteristics of gravitational, potential, and kinetic energy. Explore how energy is the driving force behind all work and movement, with examples and explanations provided for each energy type. Gain insig
2 views • 24 slides
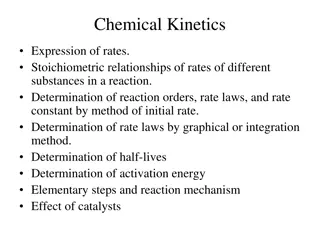
Chemical Kinetics: Understanding Reaction Rates and Factors
Chemical kinetics is a branch of physical chemistry that explores the velocity and factors influencing chemical reactions. It studies how reactants transform into products, considering conditions like temperature, pressure, and reactant concentrations. Factors affecting reaction rates include the na
7 views • 24 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Understanding Kinetic Energy Equations and Examples
Learn how to rearrange and calculate kinetic energy using the formula KE = 1/2mv^2. Explore the definition, formula representation, solving for mass and velocity, related equations, and practice with calculation examples. Understand how mass and velocity affect kinetic energy in various scenarios.
0 views • 11 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Antigen-Antibody Precipitation Reaction in Microbiology
Antigen-antibody precipitation reaction involves the formation of insoluble products when a soluble bivalent antibody interacts with a soluble antigen. This reaction leads to the formation of a visible precipitate known as a lattice. The mechanism of precipitation, including the prozone phenomenon,
0 views • 20 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Understanding Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
2 views • 68 slides
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Energy - Forms, Calculations, and Applications
Explore the concept of energy through various images, including forms of energy, kinetic versus potential energy, and calculations involving kinetic and potential energy. Learn about identifying energy states, calculating kinetic energy, and solving physics problems related to energy transfer. Dive
0 views • 27 slides
Understanding Kinetics and Reaction Rates in Chemistry
Kinetics is the study of reaction rates and factors affecting them, such as concentration, temperature, catalysts, and more. Orders of reaction classify reactions based on rate dependency on reactant concentration. Factors like pH, light, and solvents can also impact reaction rates. Half-life and sh
0 views • 18 slides
Understanding Free Radical Polymerization Kinetics
This lecture covers the kinetics of free radical polymerization, including initiation, propagation, termination, and kinetic chain length concepts. It explains the calculation of kinetic chain length and chain-transfer reactions. Key points include the rate equations for initiation, propagation, and
0 views • 11 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
Kinetic Molecular Theory and States of Matter in Physical Pharmacy
The lecture by Assistant Prof. Dr. Fouadalssady in physical pharmacy delves into the Kinetic Molecular Theory, elucidating how gases consist of particles in constant motion with negligible volume. It explains the relationship between kinetic energy, temperature, and the transition from gas to liquid
0 views • 10 slides
Understanding Potential and Kinetic Energy
Explore the difference between potential and kinetic energy, learn how they are defined, understand their conversion, and discover how they relate to speed, height, and mass in objects around us. See examples of potential and kinetic energy in action, from airplanes circling to flags blowing in the
0 views • 31 slides
Fundamentals of Asymmetric Catalysis: Energetics and Principles
Exploring the principles and energetics of asymmetric catalysis, this study delves into the importance, classes of transformations, stereoselectivity, and transmission of asymmetry. It discusses reaction coordination diagrams, transition state stabilization, and the terminology of catalysis. The Cur
2 views • 20 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
Understanding Kinetic and Potential Energy: A Visual Exploration
Delve into the concepts of kinetic and potential energy through engaging visuals and explanatory content. Learn about the factors affecting kinetic energy, compare energy levels between objects in motion, explore the calculation of kinetic energy, and discover the storage and examples of potential e
0 views • 15 slides
Understanding Chemical Reaction Kinetics in Chemcad
Explore the kinetic reactor module in Chemcad for reaction rate specification using VBA. Learn how to determine parameter values and analyze reactions such as steam reforming and water gas shift. Follow step-by-step instructions to run the unit operation, view reactor profiles, and compare reaction
0 views • 9 slides
Understanding Energy: Kinetic and Potential Explained
Explore the concepts of kinetic and potential energy through illustrations and examples. Learn how kinetic energy is influenced by mass and speed, and how potential energy can be stored in different forms such as gravitational, chemical, and elastic. Understand the relationship between mass, speed,
0 views • 12 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
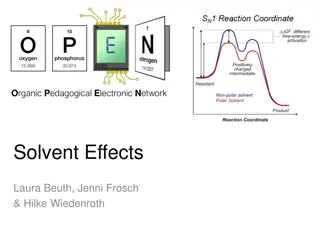
Understanding Solvent Effects in Organic Chemistry
Different solvents can have varying effects on chemical reactions by influencing solubility, stability, and reaction rates. Solvents can control the pathway towards forming either thermodynamic or kinetic products based on their selection. Solutes dissolve based on intermolecular interactions with s
0 views • 6 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Understanding Multisubstrate Enzyme Kinetic Mechanisms
In multisubstrate enzyme kinetic mechanisms, the apparent Km and Vmax values change with varying substrate concentrations. Different kinetic mechanisms like rapid equilibrium Bi Bi and ordered Bi Bi reactions can occur. The ping-pong Bi Bi reaction involves oscillation between enzyme forms. Various
0 views • 11 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Exploring Motion through Kinetic Art
Delve into the world of motion with a focus on kinetic art, inspired by the artwork of Latvian artist Valdis Celms. Discover how objects move, the forces that drive their motion, and engage in hands-on activities like creating rotating mechanisms. Through Valdis Celms' kinetic wall sculpture "Positr
0 views • 14 slides
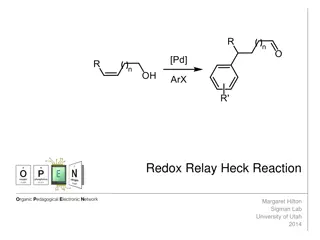
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Understanding Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Understanding Kinetic Energy and Work in Physics
Kinetic energy is the energy possessed by moving objects, allowing them to do work. When a force acts on an object causing it to displace, work is done, and the object's kinetic energy changes. The work-energy theorem states that work done on an object equals the change in its kinetic energy. Real-w
0 views • 9 slides
Understanding Kinetic and Potential Energy
Explore the relationship between potential and kinetic energy, as well as how energy causes change in objects. Learn about kinetic energy, speed, mass, and their impact on energy transfer through engaging examples and explanations.
0 views • 32 slides
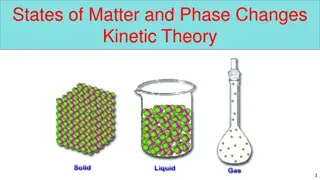
Understanding Kinetic Theory of Matter and Phases
Explore the fundamental concepts of the Kinetic Theory of Matter, including the three pillars of kinetic energy and forces of attraction, which determine the states of matter like solid, liquid, gas. Learn about temperature, phase changes, and the phases of matter, emphasizing the role of kinetic en
0 views • 17 slides
Understanding Energy: Potential and Kinetic Forms in Grade 7 Natural Sciences
Energy in various forms is explored in Grade 7 Natural Sciences, with a focus on potential and kinetic energy. Energy is the ability to do work and exists in different types like heat, chemical, electromagnetic, nuclear, and mechanical. The sun serves as a primary energy source. Potential energy is
0 views • 11 slides
Understanding Rotational Kinetic Energy and Moment of Inertia
Rotational kinetic energy arises from the motion of mass in a rotating object, while moment of inertia quantifies an object's resistance to rotational motion. This concept is crucial for analyzing the energy and stability of rotating systems. The content explains the calculation of kinetic energy fo
0 views • 7 slides
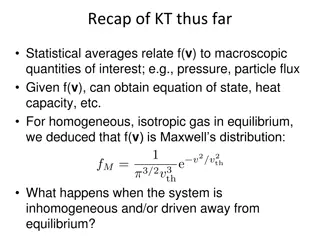
Insights into Non-equilibrium Kinetic Theory: Inhomogeneous Systems
Statistical averages in kinetic theory connect distribution functions to macroscopic properties like pressure and particle flux. When systems are inhomogeneous or away from equilibrium, local equilibrium breaks down, leading to slow relaxation processes towards global equilibrium. The evolution of p
0 views • 12 slides
Understanding Kinetic Proofreading in Biological Systems
Kinetic proofreading is a crucial mechanism by which the body accurately discriminates between closely-related molecules, such as mRNA codons and tRNA molecules in the process of protein synthesis. This process ensures that the correct molecules bind together, preventing errors that could have sever
0 views • 55 slides