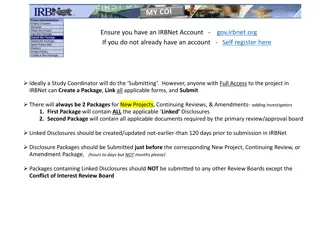
IRBNet Account Setup and COI Process Guide
Comprehensive instructions on setting up an IRBNet account, creating packages for new projects, continuing reviews, and amendments, as well as submitting linked disclosures to the Conflict of Interest Review Board. The guide also outlines the COI process for new projects, including creating a VA Pro
2 views • 19 slides
First-Time Researcher's Guide: Navigating the IRB Submission Process at Barry University IRB January 2023
Providing essential guidance for first-time researchers submitting to the Barry University IRB in January 2023. Covers initial steps such as creating an IRBNet account, completing ethics training, determining the review category, and selecting the appropriate protocol form.
0 views • 40 slides
VA Innovation and Research Review System (VAIRRS) Overview
VA Innovation and Research Review System (VAIRRS) is a platform for researchers and research personnel within the Eastern Colorado Healthcare System to submit and get approval for research studies. It runs on the IRBNet platform, where users can register and manage their projects. The system provide
0 views • 12 slides
VA Innovation and Research Review System (VAIRRS) Monthly Webinar Highlights
This monthly webinar for VA Innovation and Research Review System (VAIRRS) provides key updates and information for attendees. Topics covered include housekeeping instructions, upcoming events, important announcements such as COVID-19 research dashboard and VAEDA webinar, and a focus on IRBNet basic
0 views • 49 slides
VA Central IRB Process Changes Update
Changes in the VA Central IRB process include updates regarding IRBNet access, project ownership, submission workflow, and COI review procedures. Important points include registering for an IRBNet account, transferring project ownership to PIs or LSIs, and submitting new projects to local research a
0 views • 27 slides
Understanding IRB and IRBNet Processes at Lehman College
Explore the different types of IRB review processes, including exempt, expedited, and full/convened reviews. Learn about human subjects research, not human subjects research, and exempt categories. Discover the importance of obtaining informed consent and navigating IRBNet for research compliance at
0 views • 74 slides