Understanding IRB and IRBNet Processes at Lehman College
Explore the different types of IRB review processes, including exempt, expedited, and full/convened reviews. Learn about human subjects research, not human subjects research, and exempt categories. Discover the importance of obtaining informed consent and navigating IRBNet for research compliance at Lehman College.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Navigating the IRB & IRBNet at Lehman Tara M. Prairie Director of Responsible Research Practices Lehman College
Overview Types of Review Training www.citiprogram.org IRBNet www.irbnet.org Guiding Student Research Research NYC Department of Education
Types of Review Not Human Subjects Research Exempt Expedited Full/Convened Informed Consent
Not Human Subjects Research Research - A systematic investigation (the gathering and analysis of information) designed to develop or contribute to generalizable knowledge. Human Subject - A living individual about whom an investigator conducting research obtains 1) data through intervention or interaction with the individual, or 2) identifiable private information.
Not Human Subjects Research Publicly available dataset that does NOT require special permissions. Data about living individuals from an existing data set, where identity cannot be readily ascertained or associated with data. Class project for a grade that will not be disseminated outside the classroom. Evaluation of a program for quality improvement with NO plans to publish
Not Human Subjects Research Evaluation of a program for quality improvement with NO plans to publish or present the results outside of CUNY. Data from death records or about non-living individuals. Limited to an analysis of a single case report where findings may or may not be generalized. Open-ended interviews that ONLY document a specific historical event or the experience of individuals without the intent to draw conclusions or generalize findings.
Exempt Review Does NOT mean you are exempt from IRB review. The PI must still submit a Request for Exemption. Exempt Review status means you are exempt from continuing review (annual review) Approved for three (3) years. Must resubmit request for exemption before 3 years is up.
Exempt Category 1 Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (a) research on regular and special education instructional strategies or (b) research on the effectiveness of or the comparison among instructional techniques, curricula or classroom management methods.
Exempt Category 2 Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: a. information obtained is recorded in such a manner that subjects can be identified, directly or through identifiers linked to the subjects and b. any disclosure of the human subjects' responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects' financial standing, employability or reputation.
Exempt Category 2 The exemption at 46.101(b)(2) for research involving survey or interview procedures or observations of public behavior does not apply to research covered by Subpart D (minors), except for research involving observation of public behavior when the investigator(s) do not participate in the activities being observed.
Exempt Category 3 Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior that is not exempt under paragraph # 2 (above) if: a. The human subjects are elected or appointed public officials or candidates for public office, or b. federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter.
Exempt Category 4 Research involving the collection or study of existing data, documents, records, pathological specimens or diagnostic specimens, if these sources are publicly available OR the information is recorded by the investigator in such a manner that the subjects cannot be identified directly or through identifiers linked to the subjects.
Exempt Category 5 Research and demonstration projects which are conducted by or subject to the approval of (federal) department or agency heads and which are designed to study, evaluate or otherwise examine: (a) public benefit or service programs, (b) procedures for obtaining benefits or services under those programs, (c) possible changes in or alternatives to those programs or procedures or (d) possible changes in methods or levels of payment for benefits or services under those programs.
Exempt Category 6 Taste and food quality evaluation and consumer acceptance studies, if: a. wholesome foods without additives are consumed or b. if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the FDA or approved by the EPA or the Food Safety and Inspection Service of the U.S.D.A.
Expedited Category 1 Clinical studies of drugs and medical devices only when condition (a) or (b) is met. (a) Research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. (Note: Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for expedited review.) (b)Research on medical devices for which (i) an investigational device exemption application (21 CFR Part 812) is not required; or (ii) the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
Expedited Category 2 Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture as follows: (a) from healthy, nonpregnant adults who weigh at least 110 pounds. For these subjects, the amounts drawn may not exceed 550 ml in an 8 week period and collection may not occur more frequently than 2 times per week; or (b) from other adults and children, considering the age, weight, and health of the subjects, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected. For these subjects, the amount drawn may not exceed the lesser of 50 ml or 3 ml per kg in an 8 week period and collection may not occur more frequently than 2 times per week.
Expedited Category 3 Prospective collection of biological specimens for research purposes by noninvasive means. Examples: (a) hair and nail clippings in a nondisfiguring manner; (b) deciduous teeth at time of exfoliation or if routine patient care indicates a need for extraction; (c) permanent teeth if routine patient care indicates a need for extraction; (d) excreta and external secretions (including sweat); (e) uncannulated saliva collected either in an unstimulated fashion or stimulated by chewing gumbase or wax or by applying a dilute citric solution to the tongue; (f) placenta removed at delivery; (g) amniotic fluid obtained at the time of rupture of the membrane prior to or during labor; (h) supra- and subgingival dental plaque and calculus, provided the collection procedure is not more invasive than routine prophylactic scaling of the teeth and the process is accomplished in accordance with accepted prophylactic techniques; (i) mucosal and skin cells collected by buccal scraping or swab, skin swab, or mouth washings; (j) sputum collected after saline mist nebulization.
Expedited Category 4 Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving x- rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. (Studies intended to evaluate the safety and effectiveness of the medical device are not generally eligible for expedited review, including studies of cleared medical devices for new indications.)
Expedited Category 4 Examples: (a) physical sensors that are applied either to the surface of the body or at a distance and do not involve input of significant amounts of energy into the subject or an invasion of the subjects privacy; (b) weighing or testing sensory acuity; (c) MRI; (d) ECG, EEG, thermography, detection of naturally occurring radioactivity, ERG, ultrasound, diagnostic infrared imaging, doppler blood flow, and echo; (e) moderate exercise, muscular strength testing, body composition assessment, and flexibility testing where appropriate given the age, weight, and health of the individual.
Expedited Category 5 Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for nonresearch purposes (such as medical treatment or diagnosis).
Expedited Category 6 Collection of data from voice, video, digital, or image recordings made for research purposes.
Expedited Category 7 Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
Expedited Category 8 Continuing review of research previously approved by the convened IRB as follows: where (i) the research is permanently closed to the enrollment of new subjects; (ii) all subjects have completed all research-related interventions; and (iii) the research remains active only for long-term follow-up of subjects; or where no subjects have been enrolled and no additional risks have been identified; or where the remaining research activities are limited to data analysis.
Expedited Category 9 Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories two (2) through eight (8) do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified.
Full/Convened Review Research that cannot meet the criteria for exempt or expedited review must be submitted for full review. It can include: Vulnerable populations (when applicable) More than minimal risk (physical, psychological, social or economic).
Informed Consent Consent can be waived in certain instances. Under exempt review a Request for Waiver of Consent is not necessary.
Informed Consent A Request for Waiver of Consent can be filed for your expedited or convened study if it meets the following criteria: 1) The research involves no more than minimal risk to the subjects. 2) The waiver or alteration will not adversely affect the rights and welfare of the subjects. 3) The research could not practicably be carried out without the waiver or alteration. 4) Whenever appropriate, the subjects will be provided with additional pertinent information after participation.
A Request for Waiver of Consent CanNOT apply to: Research that involves non-viable neonates as subjects. Research that involves experimental subjects in DoD research. Pertinent research not regulated by the FDA.
Training www.citiprogram.org 1. Conflict of Interest 2. Human Subjects 3. Responsible Conduct in Research
My office only needs: Human Subjects Research Certificate Make sure to take Behavioral and Social Research as an Investigator Take the refresher course every three years
Other Research Training: Dr. Alan Kluger = Research Integrity Officer = RCR Certificate for all faculty, staff & students conducting research. Dr. Robert Troy = Conflict of Interest Officer = COI Certificate for all funded research.
IRBNet www.irbnet.org 1. Registering 2. Uploading Training 3. Starting a IRB Application
IRBNet Registration Visit www.irbnet.org Click on New Registration in the top right corner. Post Registration can click on Forgot Your Password? Fill-out the registration form following the on- screen prompts. Note Lehman sometimes shows up as Herbert H. Lehman . You will receive an account validation email from IRBNet, using the validation link provided, log into IRBNet.
Uploading CITI Training 1. Click User Profile (link at top of screen once logged in) and attach your CITI certificate by clicking Add New Record . 2. On the Training & Credentials Record page, select the document type from the drop-down menu. 3. Enter the date the certificate was effective. 4. Click Browse to locate the document that you plan to attach (once found click Open ).
Uploading CITI Training 5. Next, click Attach . 6. You should now find yourself on the User Profile page. Submit your training certificate to the IRB by clicking Submit highlighted in blue text next to the document you just added.
Uploading CITI Training 7. You will now be on the Submit Training & Credentials page. Type in Lehman in the blank and click Search . 8. Once you locate your campus name click on it and then click Continue . 9. On the new page that opens, click Submit . 10. Your document has now been submitted.
Creating a new project in IRBNet 1. After logging in, click Create New Project on left side of the page. 2. Fill out the form per the on-screen prompts. Then click Continue . 3. You will now find yourself at the Designer page. 4. Click Add New Document (Located under Step 2 of the Designer page).
Creating a new project in IRBNet 5. On the Attach Document page, next to On-Line Document (bottom of the page), select CUNY Application for Approval to use Human Subjects Part I and click Add . 6. The IRBNet Document Wizard will open and give you two options. You can clone information from previous applications or you create a new form by following the on-line prompts.
Creating a new project in IRBNet 7. Next under Step 1 of the Designer page, make sure next to Select a Library the option, CUNY Institutional Review Board Office, New York, NY is chosen. 8. From the Select a Document drop- down menu choose either Request for Exemption or Submission Form CUNY Initial Application Part II for expedited & full review research and click Download .
Creating a new project in IRBNet 9. The Select a Document drop-down menu is also where you can access consent and assent (minors 7-17) templates and any Supplement Forms you may need per Part I. 10. Save the documents to your computer and fill them out so they can be uploaded into IRBNet by clicking Add New Document in the Designer.
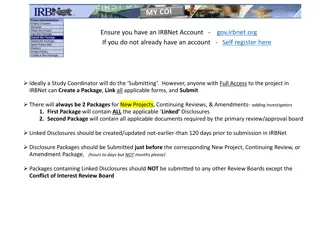
Creating a new project in IRBNet 11.Documents are uploaded by selecting an Attachment Type , e.g. Adult Consent form. 12. Click Browse to locate the document that you plan to attach (once found click Open and then click Attach. 13.After your documents are attached to your package, you can also link your CITI training to your study by clicking, link/unlink records. 14.Then you Sign and Submit (on left side of screen) the package to be reviewed for approval.
Amending or Continuing a Study in IRBNet 1. After logging in, access your project from the My Project tab on the left of the screen. 2. Click on the title of the project. 3. Click Add New Document on the Designer page. 4. After clicking, Add New Document you will be taken to a page that informs you that the Package is Locked .
Amending or Continuing a Study in IRBNet 5. On the Package is Locked page, click Create New Package . 6. You will now be on the Project History page, where a red arrow will be visible next to the new package you created. 7. Click the title of the words New Document Package highlighted in Blue.
Amending or Continuing a Study in IRBNet 8. You will now be on the Designer page for the New Package you just created. 9. Under Step 1 make sure CUNY Institutional Review Board Office, New York, NY is next to Select a Library . 10. In the drop box for Select a Document choose either the Amendment Request and/or Continuing Review Request form.
Amending or Continuing a Study in IRBNet 11.Save the documents to your computer and fill them out so they can be uploaded into IRBNet by clicking Add New Document in the Designer. 12.Documents are uploaded by selecting an Attachment Type , e.g. Adult Consent form. 13.Make sure to upload any updated forms or blank consent forms to be stamped with updated approval dates.
Amending or Continuing a Study in IRBNet 14.Click Browse to locate the document that you plan to attach (once found click Open and then click Attach. 15.After your documents are attached to your package, you can also link your CITI training to your study by clicking, link/unlink records. 16.Then you Sign and Submit (on left side of screen) the package to be reviewed for approval.
IRB Review Please note, any requested revisions should be addressed in writing and uploaded into IRBNet along with any revised documents.
Guiding Student Research IRB Guidance for Faculty Advisors
Who Can Be an Advisor? Only faculty, may serve as faculty advisors. Adjuncts and graduate teaching assistants are ineligible.
Faculty Advisors Should: Be Informed. Contact the Office of Responsible Research Practices (x8960, Shuster 303, tara.prairie@lehman.cuny.edu) to discuss policy & procedures for obtaining IRB Review before initiation of research activities. Familiarize yourself with institutional requirements for the conduct of human subjects research.