Class 2 Permit Modification Request
This Permit Modification Request (PMR) aims to transition audit scheduling for site recertification from an annual to a graded approach, incorporating DOE Orders and Quality Assurance program requirements. The PMR consolidates scheduling information, reduces redundancy, and clarifies subsequent audi
3 views • 22 slides
Utilizing Audits for Antimicrobial Stewardship in General Practice
The importance of audits in antimicrobial stewardship is highlighted in this informative content covering topics like why audit and feedback are crucial, sources of prescribing data, available audit tools, practical tips, and a case study showcasing the positive impact of audits in UTI management. T
10 views • 40 slides
Understanding Post-Election Risk-Limiting Audits in Indiana
Indiana's post-election audits, overseen by the Voting System Technical Oversight Program, utilize statistical methods to verify election outcomes, ensuring accuracy and reliability in the electoral process. The VSTOP team, led by experts in various fields, conducts audits based on Indiana Code IC 3
0 views • 12 slides
CPGP Study Guide and How to Crack Exam on Pharmaceutical GMP Professional
Click Here--- \/\/bit.ly\/4bsglWA ---Get complete detail on CPGP exam guide to crack Pharmaceutical GMP Professional. You can collect all information on CPGP tutorial, practice test, books, study material, exam questions, and syllabus. Firm your knowledge on Pharmaceutical GMP Professional and get r
3 views • 35 slides
ASQ Pharmaceutical GMP Professional (CPGP) Exam | Boost Your Score
Click Here---> \/\/bit.ly\/4bsglWA <---Get complete detail on CPGP exam guide to crack Pharmaceutical GMP Professional. You can collect all information on CPGP tutorial, practice test, books, study material, exam questions, and syllabus. Firm your knowledge on Pharmaceutical GMP Professional and get
0 views • 20 slides
Role of Supreme Audit Institution of the Philippines in Employing Artificial Intelligence to Fight Corruption
The Supreme Audit Institution of the Philippines (SAI-PHL) is utilizing technology, including artificial intelligence, to enhance its audit processes and combat corruption effectively. Through initiatives like understanding IT systems and conducting computer-assisted audits, SAI-PHL is embracing dig
0 views • 12 slides
Top PCD Pharma Franchise Company in India | WHO GMP Certified
Unibiotech Formulations is WHO GMP Certified Pharma Franchise Company in India. We offer Latest formulations in tablets, capsules, suspensions, syrup, etc. Get more info for franchise Services Contact us at 917814301804
2 views • 5 slides
Understanding GMP Audits in Construction: Navigating Client Expectations
This presentation at the National Association of Construction Auditors' virtual conference focuses on helping clients grasp the key objectives and processes of Guaranteed Maximum Price (GMP) audits. Dave Potak, a seasoned professional, will share insights on managing client expectations, best practi
0 views • 18 slides
Understanding Single Audits for Federal Fund Compliance
Explore the process and requirements of single audits for federal fund compliance, including when they are required, the responsibilities involved, and the importance of OMB Compliance Supplement. Learn how single audits provide assurance to federal agencies about fund usage compliance and the stand
1 views • 33 slides
Guidelines for Personnel Training and Hygiene in Pharmaceutical Manufacturing
Personnel responsibilities in a manufacturing unit include training, hygiene, and maintaining personal records. Guidelines as per Sch.M of D&C act 1945 outline the supervision, qualifications, and duties required for technical staff, QC lab, and QA personnel. Health, clothing, and sanitation protoco
2 views • 16 slides
Top WHO-GMP-ISO Certified Monopoly Pharma Franchise Company
Top WHO-GMP-ISO Certified Monopoly Pharma Franchise Company in India. Contact Unibiotech Formulations At 917814301804, 919216901651
1 views • 5 slides
ASEAN Guidelines on GMP for Traditional Medicines: Evaluation of Corrective Action and Preventive Action
The ASEAN Guidelines on GMP for Traditional Medicines discuss the importance of Corrective Action and Preventive Action (CAPA) for maintaining quality in health supplements. CAPA involves identifying nonconformities, implementing solutions, and preventing future occurrences through continuous improv
1 views • 47 slides
Understanding Post-Election Audits for Registrars of Voters
Post-election audits are essential for ensuring the accuracy and functionality of optical scan voting machines. This process involves randomly selecting voting districts for hand count audits to assess machine performance. The chain of custody must be strictly maintained for ballots and equipment. M
0 views • 18 slides
Understanding Wireless Security Audits and Best Practices
Explore the world of security audits with a focus on wireless networks. Learn about the types of security audits, best practices, and the steps involved. Discover the importance of systematic evaluations, identifying vulnerabilities, establishing baselines, and compliance considerations. Dive into t
0 views • 14 slides
Understanding Departmental Audits in GST
Departmental audits in GST involve the examination of records, returns, and other documents to verify the correctness of turnover declared, taxes paid, refunds claimed, and input tax credit availed. This audit ensures compliance with the provisions of the CGST Act, 2017. Types of audits under GST in
7 views • 27 slides
Guidelines for Social Audits under MGNREGA
The legal mandate for social audits under MGNREGA includes the requirement for conducting audits by Gram Sabhas, setting up independent Social Audit Units, and involving Village Social Audit Facilitators. The process involves collating records, conducting beneficiary and work verification, and prese
1 views • 19 slides
WHO-GMP, GLP, ISO Certified Pharma Manufacturing Company
Unimarck Pharma is WHO-GMP, GLP, ISO Certified Pharma Manufacturing Company Since 1984. Contact us today at 91-172-2244500.
1 views • 5 slides
Worried About IRS Audits? Here’s How SAI CPA Services Can Help You Avoid Them!
IRS audits can be stressful, but with the right preparation, you can minimize your chances of being audited. Audits often stem from discrepancies or unusual patterns in tax returns. Common triggers include math errors, large deductions, unreported in
3 views • 2 slides
Understanding Bayesian Audits in Election Processes
Bayesian audits, introduced by Ronald L. Rivest, offer a method to validate election results by sampling and analyzing paper ballots. They address the probability of incorrect winners being accepted and the upset probability of reported winners losing if all ballots were examined. The Bayesian metho
2 views • 7 slides
ASEAN Guidelines on GMP for Traditional Medicines/Health Supplements - Preparation for Inspection
This content provides guidelines on preparing for a Good Manufacturing Practice (GMP) inspection for traditional medicines and health supplements in ASEAN countries. It covers activities such as planning inspections, forming inspection teams, reviewing documentation, preparing inspection plans, hold
0 views • 19 slides
Understanding Single Audits in Federal Grant Programs
Audits play a crucial role in ensuring accountability in Federal grant programs. Single Audits, being the most common type, combine financial and compliance audits into one report. Learn about threshold determinations, risk-based approaches, and key changes in the Uniform Guidance through this compr
0 views • 26 slides
ASEAN Guidelines on GMP for Traditional Medicines - Preparation of GMP Report
The ASEAN Guidelines on GMP for Traditional Medicines provide detailed instructions on preparing GMP reports, including post-inspection activities, deficiency classification, examples of deficiencies, and inspection report format. Deficiencies are categorized as Critical, Major, or Minor, with speci
0 views • 20 slides
ASEAN Guidelines on GMP for Traditional Medicines - Philosophy of Inspection
The ASEAN Guidelines on GMP for Traditional Medicines highlight the Philosophy of Inspection for ensuring quality and safety in traditional medicines and health supplements. The document covers legal terms, audit trail requirements, electronic signature control, and familiar auditor requirements. It
0 views • 88 slides
Safety Management Overview and Audits Report
Explore a detailed report on safety management practices, audits findings, and actionable insights in the BSEE office. Learn about prior audits, SEMS evaluations, CAP verification, and more. Dive into SEMS subpart O audits and API RP 75 guidelines for a comprehensive understanding of safety protocol
0 views • 18 slides
Enhancing Feed Safety Through GMP+ International Certification
Explore the world of GMP+ International certification for feed safety, providing value to former foodstuffs. Learn how food companies can ensure safe feed practices, with a focus on compliance, incident management, and traceability. Discover the chain approach in various industries, from cultivation
0 views • 12 slides
Billing Documentation Guidelines for OSAP Programs
Audits are imminent for OSAP/BHSD programs, emphasizing the importance of proper documentation to ensure compliance and accuracy in billing. Providers must adhere to strict guidelines for submitting audits and desk audits annually, promptly informing OSAP of any staff changes. The documentation cove
0 views • 25 slides
Powercor Industry Forum Audit Results and Trends Analysis
Audit results and trends analysis reveal that there were 256 audits completed, with 50 being re-audits. Additional resources were acquired to meet industry demand, but audit volumes in Q4 did not meet forecast. Turnaround times improved with the deployment of more auditor resources. Trends show issu
0 views • 8 slides
Local Government Audit Outcomes Analysis as of February 2013
The Auditor-General of South Africa plays a crucial role in ensuring oversight, accountability, and governance in the public sector by conducting audits. This analysis reveals varying audit outcomes across provinces, highlighting the need for focused actions to improve audit results and promote clea
0 views • 22 slides
Essentials of Site Audits for Global HIV & TB Programs
Understanding the purpose, stages, and requirements of site audits is vital for ensuring accuracy and reliability in test results for Global HIV & TB programs. From identifying improvement areas to implementing corrective actions, this content provides a comprehensive guide for conducting effective
0 views • 14 slides
ASEAN Guidelines on GMP for Traditional Medicines/Health Supplements: Conducting GMP Inspection
The ASEAN Guidelines provide detailed procedures for conducting GMP inspections to ensure objectivity and appropriateness. The inspection processes include opening meetings, facility inspections, documentation review, inspector meetings, and exit meetings. During the opening meeting, the inspection
0 views • 30 slides
Conducting Surveillance of CNS Providers
Conducting surveillance of CNS providers involves audits and inspections to ensure compliance with regulatory requirements and maintain safety standards. Various types of audits, such as pre-certification and post-certification audits, are conducted by qualified CNS oversight inspectors to identify
0 views • 48 slides
Academic Audit and Importance in the Commerce Department at Shankarlal Khandelwal Arts, Science and Commerce College, Akola
The Department of Commerce (English Medium) at Shankarlal Khandelwal Arts, Science and Commerce College in Akola conducts academic audits to enhance academic standards. These audits analyze faculty and student performance, costs, and outcomes, aiding in continual improvement. The department focuses
0 views • 29 slides
Understanding the Impact of Audits on Post-Audit Tax Compliance
Audits have direct and indirect effects on taxpayers, influencing compliance behaviors. While more audits generally lead to increased compliance, outcomes can be ambiguous, with some studies showing a decline in post-audit compliance. Behavioral responses to tax audits are driven by perceived risks
0 views • 15 slides
ASEAN Guidelines on GMP for Traditional Medicines - Classification of GMP Non-Conformance
Classification of GMP non-conformance is crucial for conducting inspections and preparing reports. It helps companies take necessary actions and affects the inspection rating. The guidelines outline critical, major, minor, and other deficiencies in traditional medicines and health supplements, empha
0 views • 26 slides
Impact of Audits on Tax Compliance: Insights from Research Studies
Studies conducted by researchers such as Erich Kirchler have explored the impact of audits on tax compliance. While audits generally have a positive effect on compliance, there are cases where they can backfire, leading to unintended consequences. High auditing levels may not always deter tax evasio
0 views • 14 slides
Understanding the Interaction Between Criminal Investigations and Civil Tax Audits in Sweden
The relationship between criminal investigations and civil tax audits in Sweden is explored, highlighting how tax audits and criminal proceedings run concurrently. The mens rea requirement for criminal sanctions and tax surcharge, as well as the integration between criminal sanctions and tax surchar
0 views • 13 slides
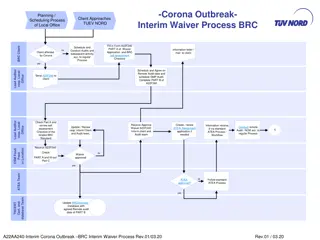
Interim Waiver Process for BRC Audits During Corona Outbreak
This content outlines the interim waiver process and scheduling procedures for local office client audits during the Corona outbreak. It includes steps for completing waiver applications, conducting remote audits, and handling certification extensions. The document also provides guidelines for remot
0 views • 11 slides
Supporting SAIs in Auditing SDGs: Reflections and Plans
SAIs play a crucial role in auditing SDGs to ensure high-quality audits of partnerships. Various SAIs and funding partners are actively involved in supporting this initiative. The story so far includes audits of preparedness and implementation of SDGs, with performance audits supporting 73 SAIs and
0 views • 14 slides
ASEAN Guidelines on GMP for Traditional Medicines - Preparation for Inspection
This content outlines the preparation activities for a Good Manufacturing Practice (GMP) inspection for Traditional Medicines/Health Supplements as specified by the ASEAN Guidelines. It covers the objectives, processes, inspection team formation, documentation review, and other key aspects involved
0 views • 19 slides
Food Industry Perspective on 3rd Party Audits and Regulatory Inspections
Overview of regulatory inspections and 3rd party audits in the food industry from the perspective of Tim Ahn, Global Director of Quality & Food Safety at Mars Chocolate. The content covers the importance of inspections, differences between inspections and audits, and the role of audits in driving qu
0 views • 12 slides