Biochemical Reactions
Exploring the basics of chemical reactions, the conservation of matter principle, exothermic and endothermic reactions, and the role of activation energy in jumpstarting reactions. Learn how elements transform to create products, the significance of energy release or absorption, and the essential co
3 views • 19 slides
Chemical equilibrium
Chemical equilibrium is a state where reactants and products reach a balance in a reaction. Learn about reversible and irreversible reactions, equilibrium constants, and how they affect chemical analysis. Discover the accuracy and applications of volumetric analysis, including titration methods like
3 views • 13 slides
Overview of Serious Adverse Reactions and Transfusion Events
This data compilation covers the reporting trends, breakdown of reports, components issued, and specific types of adverse transfusion reactions experienced within the National Healthcare Organization (NHO) from 2019 to 2022. The information includes statistics on Serious Adverse Events (SAE), Seriou
2 views • 46 slides
Understanding Equilibrium for Moving Objects
Objects can be in static equilibrium when at rest or dynamic equilibrium when moving at a constant speed. Equilibrium is maintained when there is no net force to change the state of motion. This equilibrium is possible when forces either cancel out or there is no force acting on the object. Friction
0 views • 8 slides
Overview of Organic Reactions and Mechanisms
Organic reactions can be categorized into addition, elimination, and substitution reactions, occurring through either polar or free radical mechanisms. Polar reactions may be electrophilic or nucleophilic, while free radical reactions involve radicals reacting to complete electron octets. Different
2 views • 26 slides
Understanding Chemical Equilibrium in Reversible Reactions and Laws
Chemical equilibrium in reversible reactions involves the balance between forward and backward reactions, as governed by laws like the law of mass action and the law of chemical equilibrium. These laws help in understanding the rates of reactions, equilibrium constants, and the relationship between
1 views • 12 slides
Understanding Dynamic Equilibrium in Chemical Reactions
Explanation of reversible reactions, dynamic equilibrium, and the characteristics of equilibrium in chemical systems. Covers the concept of reversible reactions, dynamic equilibrium, rules for dynamic equilibrium, and examples to illustrate these concepts visually.
0 views • 54 slides
Understanding Le Chatelier's Principle in Chemical Equilibrium
Le Chatelier's Principle states that when a system at equilibrium is disturbed by changes in concentration, temperature, or pressure, the equilibrium shifts to counteract the change. This principle can be applied to predict the direction of equilibrium when changes occur. Changes in concentration, p
0 views • 10 slides
Understanding Reaction Isotherms and Equilibrium Constants
Explore reaction isotherms and equilibrium constants through Vant Hoff's and Gibbs free energy equations. Learn about the relationship between Gibbs free energy, equilibrium constant, and temperature dependence. Discover how these concepts are applied in determining the direction of chemical reactio
1 views • 18 slides
Exploring Enzyme Kinetics for Understanding Chemical Reactions
Enzyme kinetics is a vital discipline focusing on the rate of enzyme-catalyzed reactions and how they respond to varying conditions. Reactions are classified based on reactant concentration influences. Zero, first, second, and third order reactions are distinguished, with examples like first-order r
0 views • 31 slides
Understanding Electrochemical Processes in Materials Engineering
Electrochemical processes play a crucial role in materials engineering, specifically in the context of corrosion. These processes involve both oxidation (anodic reaction) and reduction (cathodic reaction) reactions occurring simultaneously. Maintaining a balance between these reactions is essential
3 views • 22 slides
Understanding Energy Changes in Chemical Reactions
Exothermic reactions release energy to the surroundings, exhibited in processes like respiration and combustion. On the other hand, endothermic reactions absorb energy, demonstrated in examples such as photosynthesis. By observing changes in temperature and reactions between various substances, one
0 views • 24 slides
Understanding Nuclear Reactions: A Comprehensive Overview
Nuclear reactions involve direct and compound scenarios, with direct reactions occurring in a short period and compound nucleus reactions leading to long-lived excited states. Different types of reactions like elastic scattering, break-up, and compound nuclear reactions are discussed, highlighting t
5 views • 11 slides
Equilibrium of Rigid Bodies: Moments, Couples, and Forces
The topic covers the equilibrium of rigid bodies with a focus on moments, couples, and forces. It discusses concepts such as moments of a force, Varignon's theorem, types of supports, and equilibrium in two and three dimensions. The equilibrium in two-force members and three-force members is explain
0 views • 23 slides
Understanding Electrode Reactions in Electrochemistry
Exploring electrode reactions in electrochemistry involves delving into Faraday's law, coulometry, and the importance of sustainable electrode reactions. These concepts help us understand how the quantity of charge passed affects the production or consumption of substances in electrode reactions. As
4 views • 27 slides
Energy Changes in Chemical Reactions
Energy changes in chemical reactions can be categorized as exothermic and endothermic. Exothermic reactions release energy to the surroundings, while endothermic reactions absorb energy from the surroundings. Examples and uses of both types of reactions are provided, along with details on measuring
4 views • 24 slides
Understanding Hypersensitivity Reactions and Classification
Hypersensitivity reactions occur in sensitized hosts following contact with specific antigens, leading to injurious consequences. The Gell and Coombs Classification categorizes reactions into Type I, II, III, and IV based on immune response and duration. Type I reactions are immediate and humoral, w
0 views • 30 slides
Understanding Consumer Equilibrium in Economics
Consumer equilibrium refers to the point where a consumer maximizes satisfaction by spending income on commodities. In single commodity case, equilibrium is achieved when marginal utility equals price. For two commodities, equilibrium is reached when the ratio of marginal utility to price is equal.
0 views • 7 slides
Understanding Chemical Reaction Kinetics: From Unimolecular to Three-Body Reactions
Explore the fundamental concepts of chemical reactions, including unimolecular reactions like thermolysis and photolysis, bimolecular reactions, and three-body reactions. Learn about rate constants, reaction mechanisms, and the impact of pressure on reaction rates. Discover how energy transfer, phot
0 views • 9 slides
Understanding Mineral Reactions in Metamorphism
Mineral reactions play a crucial role in our comprehension of metamorphism, helping to estimate the pressures and temperatures rocks undergo. These reactions can be categorized as continuous or discontinuous, leading to different mineral products. Discontinuous reactions, exemplified by the transfor
0 views • 6 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Understanding Equilibrium Shifts and Market Reactions in Economics
Explore the impact of events on supply and demand, equilibrium shifts, new price and quantity levels, market corrections for disequilibrium, shortages, and surpluses. Learn how markets adjust to maintain balance and prices fluctuate based on changes in supply and demand. Discover the dynamics of mar
0 views • 5 slides
Understanding Chemical Equilibrium in Reversible Reactions
Chemical equilibrium occurs when the concentrations of reactants and products remain constant over time in a reversible reaction. Reaction rate is proportional to concentration, and equilibrium is reached when the rate of formation equals the rate of consumption in both directions. Reversible reacti
0 views • 25 slides
Understanding Chemical Reactions and Catalysts
Chemical reactions involve the formation of new substances from reactants, with key processes like oxidation and reduction. Reversible reactions, endothermic and exothermic reactions, and the role of catalysts in speeding up reactions are explored. The significance of chemical symbols, formulas, and
0 views • 8 slides
Understanding Nuclear Reactions: Fission, Fusion, and Energy Release
This content covers various aspects of nuclear reactions, including nuclear fission, fusion reactions, the Manhattan Project, and examples of reactions involving different particles and elements. It explains concepts like exoergic and endoergic reactions, conservation of charge and nucleon number, a
0 views • 34 slides
Understanding the Law of Mass Action in Chemical Reactions
Chemistry students often assume reactions go to completion, but the Law of Mass Action shows that equilibrium is reached with specific amounts of reactants and products. This law, demonstrated through experiments, helps determine equilibrium concentrations using the Keq expression. By applying basic
0 views • 18 slides
Insights into Persuasion and Equilibrium in Multidimensional Cheap Talk
Explore the dynamics of multidimensional cheap talk, focusing on sender-receiver interactions, influential equilibrium, welfare rankings, and fragility to asymmetries. Lessons touch on bubbling equilibrium, influential equilibrium issues, welfare rankings preferences, and the impact of asymmetric pr
0 views • 20 slides
Understanding Equilibria in Populations: Hardy-Weinberg Principle
Exploring the concept of equilibria in populations, focusing on Hardy-Weinberg principles and its implications. The discussion covers allele distributions, genotype frequencies, maintenance of equilibrium across generations, and scenarios where equilibrium may be violated. Key points include basic p
0 views • 58 slides
Understanding Energy in Chemical Reactions
Chemical reactions involve the release or absorption of energy in various forms like heat, light, sound, and electricity. Exergonic reactions release energy, while endergonic reactions absorb energy. Catalysts speed up reactions, while inhibitors slow them down without changing the amount of reactan
0 views • 8 slides
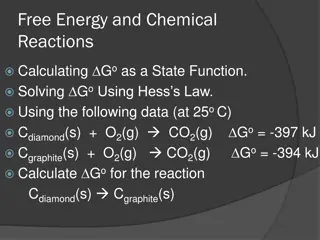
Understanding Free Energy and Chemical Reactions
Exploring concepts like Gibbs free energy, standard free energy of formation, equilibrium, and Hess's Law in chemical reactions. The feasibility of reactions, equilibrium constants, and the impact of pressure on free energy are all discussed.
0 views • 17 slides
Understanding Groundwater Sustainability and Equilibrium Dynamics
This content delves into the concepts of groundwater sustainability, equilibrium, and capture in relation to groundwater management. It explores transitional storage, groundwater mining, and debunks the water budget myth. The images and explanations illustrate how pumping affects aquifers, the evolu
0 views • 11 slides
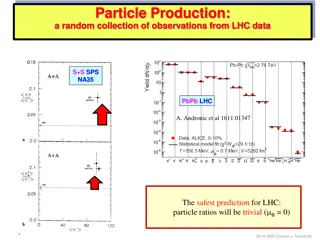
Insights into Particle Ratios and Equilibrium Dynamics at LHC
Collection of observations from LHC data regarding particle ratios and the successful Thermal Model. Discusses the concept of equilibrium, onset of equilibrium, relationship to QGP phase, and potential solutions through out-of-equilibrium studies. Also delves into size/volume dependence, strangeness
0 views • 11 slides
Understanding Homogenous Chemical Equilibrium
Homogenous chemical equilibrium occurs when reactants and products are in the same phase. This equilibrium remains independent of the volume of the reaction mixture. The concept is illustrated through the example of the Hydrogen-Iodide system and a generic reaction A + B --> 2C. Partial pressure pla
0 views • 56 slides
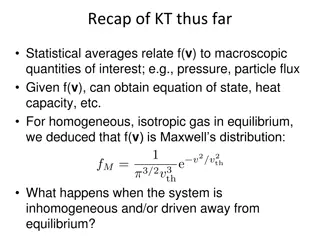
Insights into Non-equilibrium Kinetic Theory: Inhomogeneous Systems
Statistical averages in kinetic theory connect distribution functions to macroscopic properties like pressure and particle flux. When systems are inhomogeneous or away from equilibrium, local equilibrium breaks down, leading to slow relaxation processes towards global equilibrium. The evolution of p
0 views • 12 slides
Understanding Equilibriums in Physics
Equilibrium in physics refers to the state of a body where there is no change in translational or rotational motion. This state can be classified into static equilibrium (when total force and torque are zero) and dynamic equilibrium (when a body is in uniform motion with zero resultant force and tor
0 views • 9 slides
Understanding Market Equilibrium
Market equilibrium is achieved when the quantity demanded equals the quantity supplied at a specific price, ensuring a balance in the marketplace. Demand and supply schedules play a crucial role in determining market equilibrium, with excess supply or demand occurring when prices deviate from the eq
0 views • 12 slides
Understanding Game Abstraction and Equilibrium
Extensive-Form Game Abstraction with Bounds delves into the complexities of game abstraction, exploring theoretical guarantees, algorithmic challenges, and equilibrium-finding processes. The difficulty of game abstraction is examined, highlighting issues such as pathologies and the struggle to optim
0 views • 22 slides
Understanding Chemical Equilibrium in Chemistry
Exploring the concept of chemical equilibrium in chemistry, where reactions can occur in both forward and reverse directions to an appreciable extent. Learn about basic equilibrium principles, equilibrium problems, manipulating reactions, and common example problems in nomenclature. Understand how r
0 views • 24 slides
Equilibrium and Acid-Base Problems in Chemistry Lecture
In this lecture, topics such as Advanced Equilibrium, Acid/Base Equilibria, Systematic Method for solving chemical problems, Strong Acid/Strong Base scenarios, and General Comments on reactions are discussed. Examples using the systematic method are provided for practical understanding. Key points o
0 views • 13 slides
Understanding Redox Half Reactions: Assigning Oxidation Numbers and Half-Reactions
In this content, we explore assigning oxidation numbers to elements in compounds such as HClO, HClO2, HClO3, and HClO4. We then delve into the Haber Process to understand redox reactions. The concept of oxidation and reduction, as well as the significance of electrons in these reactions, is illustra
0 views • 20 slides