Research at Hughes Spalding. Updated Review and Approval Process
Navigate the research review and approval process efficiently as a busy clinician investigator at Hughes Spalding with insights on key responsibilities, approval levels, and contact information of relevant personnel. From pre-award tasks like facilitating reviews and gaining approvals to post-award
3 views • 6 slides
Huntington's Disease Research Opportunities with Dr. Jamie Herron
Dr. Jamie Herron, a Consultant Psychiatrist specializing in Huntington's Disease, presents research opportunities including sub-investigator roles in clinical trials like Wave SNP3 and biomarker studies. The portfolio outlines the genetic basis of Huntington's, associated symptoms, and the chance to
2 views • 6 slides
Overview of UW Clinical Trial Office Budget Review
UW Clinical Trial Office conducts budget reviews to ensure compliance and financial accountability in clinical trials. The office collaborates with various departments to manage billing compliance, financial risks, and institutional policies. The primary focus is on avoiding patient billing errors,
0 views • 17 slides
India Alliance Clinical & Public Health fellowship in India
India Alliance Clinical & Public Health fellowship in India\n\nIndia Alliance Clinical and Public Health Research Fellowships are for Health researchers with an MD, MS, MPH, or an equivalent clinical or public health degree, who can apply for the DBT\/Wellcome Trust India Alliance Clinical and Publi
0 views • 5 slides
Clinical Escalation: Building Effective Communication in Maternity Units
Exploring the importance of clinical escalation in maternity units, this session outlines the components and practices involved in identifying, communicating, and acting upon clinical concerns. It emphasizes recognizing deviation from normality, effective communication, and taking appropriate action
0 views • 27 slides
Ethical Issues in Clinical Pharmacy Research by Dr. Haider Raheem Mohammad
Research ethics play a crucial role in clinical trials and therapeutic research in the field of pharmacy. From discovery to validation, all medicines undergo rigorous evaluation processes to ensure safety, efficacy, and freedom from adverse effects. Clinical trials in both animals and humans are ess
0 views • 20 slides
Understanding Regulatory Requirements of Drugs and Pharmaceuticals
Drug regulation involves controlling drug use through international agreement authorities like the FDA, EMA, and PMDA. The FDA plays a crucial role in drug evaluation and research, biologic evaluation, devices, and food safety. There are various types of applications for drug approval, along with a
0 views • 28 slides
Advanced Clinical Practice Framework and Pillars of Practice
The document discusses the advanced clinical practice framework and the four pillars of practice which include leadership & management, clinical practice, education, and research. It emphasizes the importance of core capabilities and area-specific competence in advanced clinical practice. The role o
2 views • 8 slides
Objective Structured Clinical Examination (OSCE): A Modern Approach to Assessing Clinical Competence
The Objective Structured Clinical Examination (OSCE) is a modern examination method widely used in the field of health science to evaluate clinical skill performance. It involves stations where medical students interact with simulated patients to demonstrate competencies such as history taking, phys
1 views • 40 slides
NIMH Clinical Research Education and Monitoring Program Overview
NIMH's Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) aims to ensure the proper conduct, recording, and reporting of clinical trials. This program includes clinical monitoring plans, guidelines for site monitoring activities, and independent clinical monit
1 views • 29 slides
Understanding Non-Clinical Development in Therapeutic Innovation
The European Patients Academy on Therapeutic Innovation focuses on the non-clinical development phase of medicine, delving into efficacy assessment, safety evaluation, and manufacturing process considerations. Non-clinical studies are essential for decision-making in clinical trials, marketing appli
1 views • 26 slides
Ensuring Protocol Compliance and Corrective Action Plans in Clinical Trials
This content discusses the importance of creating Corrective Action and Preventive Action (CAPA) plans for protocol deviations in clinical trials. It covers the components of a CAPA, best practices for creating CAPAs for different deviation types, and regulatory compliance requirements according to
0 views • 33 slides
REDCap Cloud: Advancing Clinical Trials with Enhanced Features
Streamline your clinical trial processes with REDCap Cloud, a secure and validated platform offering improved user interface, pre-configured ePRO, and compliance with regulations. Benefit from free licensing for investigator-initiated trials and explore the easy setup steps for creating forms and ev
0 views • 11 slides
Investigator Roles and Responsibilities in Clinical Research
Key responsibilities of investigators in clinical research involve ensuring participant safety, compliance with regulations, accurate data collection, and ethical conduct throughout the study process. Properly trained investigators play a crucial role in the success and integrity of clinical trials.
2 views • 81 slides
Principal Investigator Responsibilities in Clinical Trials
The Principal Investigator (PI) plays a crucial role in conducting clinical trials. Responsibilities include overseeing the trial at the site, making critical decisions, ensuring compliance with protocols, obtaining informed consent, maintaining accurate records, and more. Non-compliance can lead to
0 views • 36 slides
Understanding Evidence-Based Medicine and Clinical Decision-Making
European Patients Academy on Therapeutic Innovation emphasizes the importance of Evidence-Based Medicine (EBM) in providing optimum clinical care. EBM involves systematic review and utilization of clinical research for informed decision-making, benefiting patients in disease management and treatment
7 views • 20 slides
Essential Aspects of the Clinical Interview in Psychology
Clinical interviews play a crucial role in the assessment conducted by clinical psychologists, showcasing essential qualities like validity, reliability, and clinical utility. Understanding the importance of feedback and honing general and specific skills as an interviewer are key components in cond
1 views • 17 slides
Rural Access Compliance Rules Proposal by Glenn Disher - PBM Investigator
Proposal by Glenn Disher, a PBM Compliance Investigator, outlines rules for rural access compliance. The proposal focuses on considering local conditions and enforcing rules for maximum impact. It includes recommendations for zip code rules, compliance mileage rules, and examples of non-compliant ru
0 views • 7 slides
Clinical Research Guidelines and Regulations Overview
Clinical research encompasses various guidelines and regulations to ensure the protection of human subjects and the credibility of study results. Key aspects include Good Clinical Practice (GCP) standards, Title 45 of the Code of Federal Regulations (CFR) Part 46, and additional CFR sections for cli
0 views • 46 slides
Ohio Clinical Alliance: Transforming Clinical Experiences
The Ohio Clinical Alliance, through collaborative partnerships, aims to enhance clinical preparation for educators. The leadership team comprises various representatives and organizations committed to improving student learning. Their activities include retreats and meetings to ensure effective comm
0 views • 27 slides
Sponsor Expectations for Clinical Studies at Thomas Jefferson University
This presentation discusses the sponsor expectations for clinical studies, investigator responsibilities, TJU's strengths in conducting studies, and how principal investigators can impress sponsors. Topics include assessment of investigator performance, regulatory guidelines, streamlining processes,
0 views • 11 slides
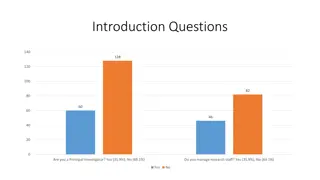
Survey Results on Research Training and Educational Support in Clinical Research Settings
This presentation showcases survey data on various aspects of research training and educational support in clinical research environments, including questions on being a Principal Investigator, managing research staff, training adequacy, study types, tenure at the University of Chicago, training pre
0 views • 35 slides
Hire the Best Private Investigator in Malibu at Kinsey Investigations
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
Hire the Best Private Investigator in Malibu
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
Choose The Best Private Investigator in Malibu
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
MUSC College of Medicine Promotion and Tenure Process
The MUSC College of Medicine outlines the detailed process and criteria for appointments, promotions, and tenure. It involves submission of packets to committees, reviews by subcommittees and full committees, and final recommendations for approval or disapproval. Specific criteria are provided for f
0 views • 28 slides
Guidelines for Academic Promotions in Medical School
Academic promotions in a medical school are crucial for recognizing achievements, maintaining competitiveness, and serving the institution's interests. Promotion criteria include teaching effectiveness, scholarly activity, clinical service, and active participation in various communities. Meritoriou
0 views • 47 slides
Private Investigator in Malibu
Seeking a reliable Private Investigator in Malibu? Kinsey Investigations specializes in discreet and effective solutions for all your investigative needs. Contact us today for professional assistance you can trust.
0 views • 6 slides
CSP Budget Request Instructions for Fiscal Year 2020
Detailed instructions for completing the CSP Request for Budgetary Support form for cooperative studies coordinated by the CSP for Fiscal Year 2020, emphasizing the completion and approval process, personnel costs estimation, clinical care funding requirements, and investigator status indication.
0 views • 31 slides
Perception and Awareness of Clinical Research in Trial Participants and the Public of Andhra Pradesh
This study focuses on understanding the perception and awareness of clinical research among trial participants and the general public in Andhra Pradesh. It highlights the importance of creating awareness about clinical research, previous study results, public attitudes towards clinical trials, and e
0 views • 24 slides
Update on PQIP Programme Progress and Future Plans
Ramani Moonesinghe, Chief Investigator of PQIP, provides an update on the programme's recruitment statistics, changes to the protocol and dataset, upcoming events like the annual report and HSRC conference, as well as future plans for clinical trials and quality improvement initiatives. The focus is
0 views • 16 slides
Quality Issues in Clinical Trial Materials: CMC Review by Dr. Dorota Matecka
Clinical trial materials undergo Chemistry, Manufacturing, and Controls (CMC) review to ensure pharmaceutical quality. This process includes assessing safety concerns, impurities, and specifications, along with other CMC considerations. Pharmaceutical quality encompasses the suitability, identity, s
0 views • 41 slides
Understanding Pharmacology and Toxicology in Investigator Brochures
Explore the essential aspects of pharmacology and toxicology covered in Investigator Brochures, including nonclinical information, safety pharmacology, general toxicology, genetic toxicology, and more. Learn about the significance of pharmacology in predicting intended and unintended effects, consid
1 views • 40 slides
Comparison of Professional Behaviors in Clinical Education
Professional behavior characteristics play a crucial role in enhancing student learning during clinical education. This study examines the differences in reported importance and frequency of professional behaviors between credentialed and non-credentialed clinical instructors. The background outline
0 views • 28 slides
Enhancing Clinical Academic Collaboration Between Universities and NHS Trusts
Clinical academics play a crucial role in integrating clinical practice, research, and education within the NHS. Collaboration between universities and NHS trusts is key to ensure clinical academics address the right questions for patient care and societal benefit. Challenges include an aging clinic
0 views • 29 slides
Insights from CTTI ICH E6 Survey and Stakeholder Input
Explore key findings from the Clinical Trials Transformation Initiative (CTTI) ICH E6 Survey, identifying areas in need of updating and stakeholder perspectives. Survey participants, from various regions, emphasized updating top ICH E6 principles like quality assurance systems and informed consent.
0 views • 21 slides
Clinical Investigator Perspectives on Lung Cancer Management: Live CME Event
Clinical investigators including D. Ross Camidge, Stephen V. Liu, Solange Peters, Gregory J. Riely, and David R. Spigel share their insights on lung cancer management at a live CME event in Chicago. The moderator, Neil Love, discloses ties with various commercial interests for educational grants. Th
0 views • 9 slides
NHMRC's New Grant Program: Advancing Clinical Trials and Research Funding
The NHMRC's latest grant program aims to enhance research in healthcare by focusing on clinical trials funding across four key streams: Investigator Grants, Synergy Grants, Ideas Grants, and Strategic and Leveraging Grants. The redistribution of funding will see a significant increase in support for
0 views • 12 slides
Challenges and Concerns in Immunology Research Funding: Investigator's Perspective
Investigator's face challenges in understanding NIH funding priorities, decreases in basic science grants, disease-earmarked funding changes, application logistics confusion, grant funding mechanisms variations, new rigorous research requirements, and equity in grant budgets.
0 views • 17 slides
Understanding Clinical Trials: Phases, Types, and Definitions
Clinical trials play a crucial role in advancing medical research and treatment options. This comprehensive guide covers the basics of clinical trials, including their definition, phases, types, and key definitions like IND, IDE, NDA, and more. Discover how different phases of trials work, the vario
0 views • 16 slides