OCTIVUS Randomized Clinical Trial: OCT-Guided vs IVUS-Guided PCI
The OCTIVUS Randomized Clinical Trial compared the clinical efficacy and safety of Optical Coherence Tomography (OCT)-guided and Intravascular Ultrasound (IVUS)-guided strategies in patients undergoing PCI for significant CAD. The study aimed to determine if OCT-guided PCI is noninferior to IVUS-gui
8 views • 27 slides
ARREST Trial: Expedited Transfer for Non-ST Elevation OHCA
This prospective, multicentre, randomised clinical trial led by Dr. Tiffany Patterson aims to determine whether direct delivery to a cardiac arrest centre following non-ST elevation out-of-hospital cardiac arrest reduces deaths compared to delivery to the nearest emergency department. Funded by the
0 views • 17 slides
Efficacy and Safety of Ferric Carboxymaltose for Heart Failure with Iron Deficiency
Iron deficiency is prevalent in heart failure patients with reduced ejection fraction and is linked to poor outcomes. The HEART-FID trial investigates the impact of intravenous ferric carboxymaltose (FCM) on all-cause mortality, heart failure hospitalizations, and exercise capacity in chronic HFrEF
1 views • 17 slides
India Alliance Clinical & Public Health fellowship in India
India Alliance Clinical & Public Health fellowship in India\n\nIndia Alliance Clinical and Public Health Research Fellowships are for Health researchers with an MD, MS, MPH, or an equivalent clinical or public health degree, who can apply for the DBT\/Wellcome Trust India Alliance Clinical and Publi
0 views • 5 slides
Ethical Issues in Clinical Pharmacy Research by Dr. Haider Raheem Mohammad
Research ethics play a crucial role in clinical trials and therapeutic research in the field of pharmacy. From discovery to validation, all medicines undergo rigorous evaluation processes to ensure safety, efficacy, and freedom from adverse effects. Clinical trials in both animals and humans are ess
0 views • 20 slides
Advanced Clinical Practice Framework and Pillars of Practice
The document discusses the advanced clinical practice framework and the four pillars of practice which include leadership & management, clinical practice, education, and research. It emphasizes the importance of core capabilities and area-specific competence in advanced clinical practice. The role o
2 views • 8 slides
Digital Health Technology-Derived Clinical Outcome Assessments in Regulatory Decision-Making
This session discusses the landscape of DHT-derived novel endpoints in clinical research, focusing on considerations for regulatory decision-making. It explains the use of digital health technology in clinical outcome assessments and highlights the potential benefits of digitally collected COAs, suc
0 views • 42 slides
Objective Structured Clinical Examination (OSCE): A Modern Approach to Assessing Clinical Competence
The Objective Structured Clinical Examination (OSCE) is a modern examination method widely used in the field of health science to evaluate clinical skill performance. It involves stations where medical students interact with simulated patients to demonstrate competencies such as history taking, phys
1 views • 40 slides
NIMH Clinical Research Education and Monitoring Program Overview
NIMH's Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) aims to ensure the proper conduct, recording, and reporting of clinical trials. This program includes clinical monitoring plans, guidelines for site monitoring activities, and independent clinical monit
1 views • 29 slides
Understanding Statistical Methods for Clinical Endpoints in Diabetes Research
This educational slide module delves into fundamental statistics for analyzing clinical endpoints in diabetes research. It covers the choice of statistical methods, the distinction between statistical and clinical significance, and the importance of different endpoints in evaluating clinical benefit
1 views • 37 slides
Comparison of FFR-guided PCI vs Angiography-guided PCI in AMI with Multivessel Disease: FRAME-AMI Trial
In patients with acute myocardial infarction (AMI) and multivessel coronary artery disease, this study aims to compare fractional flow reserve (FFR)-guided PCI with angiography-guided PCI for non-infarct-related artery lesions. The hypothesis is that selective PCI guided by FFR is superior to routin
2 views • 23 slides
Understanding Non-Clinical Development in Therapeutic Innovation
The European Patients Academy on Therapeutic Innovation focuses on the non-clinical development phase of medicine, delving into efficacy assessment, safety evaluation, and manufacturing process considerations. Non-clinical studies are essential for decision-making in clinical trials, marketing appli
1 views • 26 slides
Digital Differential Analyzer (DDA) Algorithm in Computer Graphics
In computer graphics, the Digital Differential Analyzer (DDA) Algorithm is utilized as the basic line drawing algorithm. This method involves interpolation of variables between two endpoints to rasterize lines, triangles, and polygons efficiently. The algorithm requires inputting coordinates of two
0 views • 9 slides
Understanding Biomarkers in Therapeutic Innovation
European Patients Academy on Therapeutic Innovation explains how biomarkers are measurable indicators providing insights into health conditions. Biomarkers like glucose levels in diabetes management and MRI imaging for Multiple Sclerosis play crucial roles. The Academy aims to enhance the medicine d
0 views • 24 slides
Understanding Evidence-Based Medicine and Clinical Decision-Making
European Patients Academy on Therapeutic Innovation emphasizes the importance of Evidence-Based Medicine (EBM) in providing optimum clinical care. EBM involves systematic review and utilization of clinical research for informed decision-making, benefiting patients in disease management and treatment
7 views • 20 slides
New FDA Guidance on Early Alzheimer's Disease - Janice Hitchcock, Ph.D.
Janice Hitchcock, Ph.D., discussed the new FDA guidance on early Alzheimer's disease in a webinar. The guidance highlights key sections such as introduction, background, and diagnostic criteria. It emphasizes the importance of early intervention, appropriate outcome measures, and diagnostic criteria
0 views • 13 slides
Essential Aspects of the Clinical Interview in Psychology
Clinical interviews play a crucial role in the assessment conducted by clinical psychologists, showcasing essential qualities like validity, reliability, and clinical utility. Understanding the importance of feedback and honing general and specific skills as an interviewer are key components in cond
1 views • 17 slides
Challenges and Recommendations for DHT-derived Endpoints in Clinical Trials
This study by CTTI explores barriers and solutions for adopting digitally derived endpoints in clinical trials. Through in-depth interviews with industry sponsors, the research identifies gaps, barriers, and recommendations for using DHT-derived novel endpoints as key endpoints in pivotal clinical t
0 views • 16 slides
Clinical Research Guidelines and Regulations Overview
Clinical research encompasses various guidelines and regulations to ensure the protection of human subjects and the credibility of study results. Key aspects include Good Clinical Practice (GCP) standards, Title 45 of the Code of Federal Regulations (CFR) Part 46, and additional CFR sections for cli
0 views • 46 slides
Advances in Aortic Valve Development and TAVR Trials
The presentation discusses various endpoints in aortic valve development and Transcatheter Aortic Valve Replacement (TAVR) trials, including comparisons, study stages, and newer devices. It covers different trial populations, primary and secondary endpoints, and the evaluation of non-inferiority. Su
0 views • 18 slides
Comparison of Valve Types and Anesthesia Strategies in Transcatheter Aortic Valve Implantation
In the SOLVE-TAVI trial, patients undergoing transcatheter aortic valve implantation were randomized to receive either self-expandable or balloon-expandable valves under general or local anesthesia. The study aims to compare the effectiveness and safety of different valve types and anesthesia approa
0 views • 27 slides
Ohio Clinical Alliance: Transforming Clinical Experiences
The Ohio Clinical Alliance, through collaborative partnerships, aims to enhance clinical preparation for educators. The leadership team comprises various representatives and organizations committed to improving student learning. Their activities include retreats and meetings to ensure effective comm
0 views • 27 slides
ADNI Private Partner Scientific Board (PPSB) Update 2015
The ADNI Private Partner Scientific Board (PPSB) Update for 2015 highlights key leadership, partner organizations, deliverables, and accomplishments in advancing drug development. Susan De Santi as Chairperson, along with industry leaders, plays a crucial role in advising and collaborating with ADNI
0 views • 23 slides
Understanding the Importance of Validity, Reliability, and Reproducibility in Clinical Measurements
Clinical practice involves measuring quantities to aid in diagnosis, predict patient outcomes, and serve as study endpoints. Errors in measurements can lead to inaccurate results and affect clinical decisions. Trueness and precision, accuracy, bias, and method comparison are essential concepts in as
0 views • 25 slides
Exploration of Endpoints in Clinical Trials for Alcohol Use Disorder
This presentation discusses the exploration of endpoints in pivotal clinical trials to treat Alcohol Use Disorder (AUD), focusing on Primary Alcohol Drinking Endpoints like PSNHDD. Evidence supporting PSNHDD as a primary endpoint includes clinical benefit data from alcohol clinical trials and epidem
0 views • 54 slides
Perception and Awareness of Clinical Research in Trial Participants and the Public of Andhra Pradesh
This study focuses on understanding the perception and awareness of clinical research among trial participants and the general public in Andhra Pradesh. It highlights the importance of creating awareness about clinical research, previous study results, public attitudes towards clinical trials, and e
0 views • 24 slides
Understanding Pain, Distress, and Humane Endpoints in Animal Research
Explore the concepts of pain, distress, and humane endpoints in animals through a training program aimed at researchers developing protocols for the welfare of laboratory animals. The program includes pre- and post-assessments to gauge knowledge and learning outcomes, covers scenarios illustrating c
0 views • 19 slides
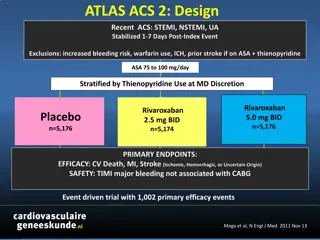
Clinical Trial Results of Rivaroxaban in ACS Patients: ATLAS-ACS 2 Study
The ATLAS-ACS 2 study investigated the efficacy and safety of rivaroxaban in ACS patients post-index event. The primary endpoints included cardiovascular death, MI, and stroke, with significant reductions seen with rivaroxaban compared to placebo. Stent thrombosis was also reduced with rivaroxaban t
0 views • 10 slides
Overview of PaNOSC Workshop API Endpoints & Data Structures
This project discusses the API endpoints and data structures from the PaNOSC Workshop, focusing on reviewing use cases, required search results/data, actors involved, and the design of endpoints reflecting proposal/experiment processes. It covers detailed discussions on data models, instrument setup
0 views • 19 slides
Comparison of Professional Behaviors in Clinical Education
Professional behavior characteristics play a crucial role in enhancing student learning during clinical education. This study examines the differences in reported importance and frequency of professional behaviors between credentialed and non-credentialed clinical instructors. The background outline
0 views • 28 slides
Understanding Digital Health Technology-Derived Clinical Outcome Assessments
This presentation delves into digital health technology-derived clinical outcome assessments (COAs) and their significance in regulatory decision-making. Exploring the definition of digital health technology (DHT), the focus on digitally collected COAs, examples from consortium workshops, and potent
0 views • 12 slides
Algorithm for Determining Endpoints in Speech Recognition
This article discusses an algorithm proposed by L.R. Rabiner and M.R. Sambur in 1975 for determining endpoints in isolated utterances. The algorithm focuses on detecting word boundaries in speech through the recognition of silence, which can lead to reduced processing load and increased convenience,
0 views • 22 slides
IGEL Universal Management Suite - Efficient Endpoint Control Solution
IGEL Universal Management Suite (UMS) is a comprehensive system that empowers IT professionals with easy control over all endpoints. Included features like centralized installation, configuration, and update management make UMS a versatile tool for managing IGEL Zero Clients and third-party endpoint
0 views • 25 slides
Enhancing Clinical Academic Collaboration Between Universities and NHS Trusts
Clinical academics play a crucial role in integrating clinical practice, research, and education within the NHS. Collaboration between universities and NHS trusts is key to ensure clinical academics address the right questions for patient care and societal benefit. Challenges include an aging clinic
0 views • 29 slides
Understanding Clinical Trial Endpoints and Regulatory Basis
Eugene J. Sullivan, MD, explains the importance of establishing efficacy in clinical trials based on substantial evidence from well-controlled trials. The discussion includes primary endpoints for Phase 3 trials, legal and regulatory requirements, and the significance of clinically meaningful effect
0 views • 21 slides
Guidelines for Scientific Endpoints and Humane Interventions in Animal Research
These guidelines outline the identification of scientific endpoints, humane intervention points, and cumulative endpoints in animal research. They emphasize the need for protocols approved by animal care committees, consideration of welfare-appropriate endpoints, and adaptation of endpoints during t
0 views • 17 slides
Veronika Logovinsky, MD, PhD - PPSB Chairperson Update 2017
Veronika Logovinsky, MD, PhD, chaired the ADNI Private Partner Scientific Board (PPSB) meeting on July 14, 2017. The PPSB, comprising leadership and working groups, focuses on providing advice for ADNI 3 implementation and accelerating drug development. The Clinical Endpoints Working Group, led by V
0 views • 21 slides
Phase II Trial Design in Oncology Drug Development by Wendy R. Parulekar, MD, FRCP
This presentation covers the pivotal role of Phase II trials in oncology drug development, emphasizing the importance of preliminary clinical efficacy screening, adverse event profiling, mechanism of action understanding, and target population definition. It discusses various Phase II trial designs,
0 views • 76 slides
Understanding Clinical Trials: Phases, Types, and Definitions
Clinical trials play a crucial role in advancing medical research and treatment options. This comprehensive guide covers the basics of clinical trials, including their definition, phases, types, and key definitions like IND, IDE, NDA, and more. Discover how different phases of trials work, the vario
0 views • 16 slides
Understanding and Minimizing Pain and Distress in Research Protocols
This content explores the importance of reviewing research protocols to minimize pain and distress in animal experiments. It covers defining pain, distress, experimental and humane endpoints, evaluating management strategies, and ensuring ethical practices. It also includes a quick exercise to asses
0 views • 11 slides