Crystal Field Theory in Transition Metal Complexes
Crystal Field Theory (CFT) explains the colors and magnetic properties of transition metal complexes. It focuses on the energy changes in d-orbitals of metal ions caused by surrounding ligands. This theory, developed in 1929, provides insights into the bonding interactions in complex compounds. The
10 views • 44 slides
Understanding Atomic Configurations and Term Symbols
The energy of atomic configurations is determined by electrostatic attraction between electrons and the nucleus, electron-electron repulsion, spin-orbit coupling, and spin-spin interactions. Term symbols in electronic spectroscopy specify atomic states using quantum numbers. Hund's rule and the Paul
8 views • 12 slides
Understanding Hyperfine Interactions in Atomic Physics
Hyperfine interactions play a crucial role in atomic physics, leading to small energy shifts and splitting of degenerate levels in atoms and molecules. These interactions involve the electromagnetic multipole interactions between the nucleus and electron clouds, resulting in the splitting of energy
13 views • 154 slides
Understanding Atomic Structure: The Evolution of Atomic Theories
Explore the journey of atomic theory from Democritus to Dalton and Thomson, uncovering the discoveries and concepts that shaped our understanding of the building blocks of matter. From the indivisible atoms proposed by Democritus to Dalton's theory of elements and compounds, and Thomson's experiment
6 views • 36 slides
Unveiling the Journey of Atomic Structure Evolution
Delve into the historical perspectives and key theories that shaped our understanding of atomic structure. From Democritus' concept of indivisible atoms to Dalton's atomic theory and Thomson's discoveries on electric charges, this journey explores the evolution of atomic theory through the insights
0 views • 35 slides
Exploring Quantum Theory and the Atom: Electrons in Atoms and the Periodic Table
Delve into the fascinating world of quantum theory and the atom in Chapter 9, where we compare Bohr's model with the quantum mechanical model. Understand de Broglie's wave-particle duality and Heisenberg's uncertainty principle's impact on our current electron view. Discover the relationships among
0 views • 31 slides
Understanding Hybridization in Organic Chemistry
Delve into the complexities of the Lewis octet model and the insights provided by Linus Pauling's localized valence bond hybridization model to explain bond shapes in molecules, reactivity trends, and electron distribution in double and triple bonds. Discover how hybridization transforms atomic orbi
0 views • 22 slides
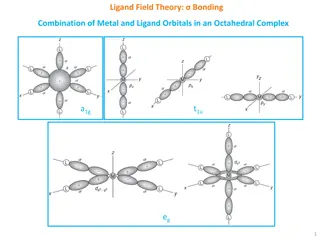
Understanding Ligand Field Theory in Octahedral Complexes
Ligand Field Theory explains the bonding interactions between metal and ligand orbitals in octahedral complexes. This theory involves the combination of metal and ligand orbitals to form molecular orbitals, leading to specific electronic configurations. The overlap of metal and ligand group orbitals
1 views • 10 slides
Understanding Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Understanding Atomic Properties and Covalent Radii in Chemistry
Exploring the concept of atomic properties including the sizes of atoms and ions, the three common operational radius concepts (covalent, crystal, and van der Waals), and the calculation of covalent radius for homonuclear and heteronuclear diatomic molecules. This overview delves into the significan
1 views • 10 slides
Understanding Atomic Absorption Spectrophotometry in Analytical Chemistry
Atomic absorption spectrophotometry (AAS) is a spectro-analytical technique used for quantitative determination of chemical elements through the absorption of light by free atoms. This method is vital in various fields like biophysics, toxicology, and archaeology, allowing the analysis of over 70 di
0 views • 9 slides
Understanding Atomic Structure and Subatomic Particles
Delve into the world of atomic structure and subatomic particles to reveal the inner workings of elements. Discover how to determine atomic mass, identify protons, neutrons, and electrons, and interpret the periodic table. Explore the key concepts of isotopes, electron configurations, and the charac
0 views • 6 slides
Understanding Atomic Structure: Electrons, Energy Levels, and Historical Models
The atomic model describes how electrons occupy energy levels or shells in an atom. These energy levels have specific capacities for electrons. The electronic structure of an atom is represented by numbers indicating electron distribution. Over time, scientists have developed atomic models based on
0 views • 5 slides
Understanding Quantum Mechanics in Atomic Structure
Exploring the connection between quantum mechanics and the fundamental elements of the periodic table, this material delves into the Schrödinger equation, quantization of angular momentum and electron spin, and the implications on atomic structure. The content covers writing the Schrödinger equati
1 views • 32 slides
Understanding Covalent Bonds and Molecular Structure in Organic Chemistry
The neutral collection of atoms in molecules held together by covalent bonds is crucial in organic chemistry. Various structures like Lewis and Kekulé help represent bond formations. The concept of hybridization explains how carbon forms tetrahedral bonds in molecules like methane. SP3 hybrid orbit
0 views • 4 slides
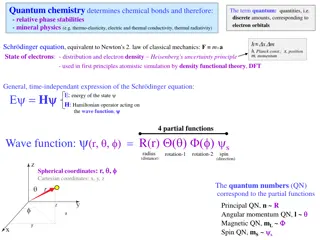
Understanding Quantum Chemistry and Electron Orbitals
Quantum chemistry plays a key role in determining chemical bonds, phase stabilities, and mineral physics through the study of electron orbitals, quantum numbers, and energy levels. This involves concepts such as the Schrödinger equation, quantum quantities, and the uncertainty principle. The arrang
0 views • 31 slides
Understanding Periodic Trends: Atomic Radius and Ionization Energy
The Periodic Table displays a systematic organization of elements based on their increasing atomic number, leading to periodic patterns in their physical and chemical properties. Key trends like Atomic Radius and Ionization Energy provide insights into the size of atoms and the energy required to re
1 views • 13 slides
Understanding Atomic Structure, Elements, Mixtures, and Compounds
Exploring the basics of atomic structure, including elements, mixtures, and compounds. Learn about single atoms, molecules of elements, and compounds formed by different elements. Understand the relationship between protons, neutrons, electrons, atomic number, and atomic mass. Test your knowledge on
0 views • 6 slides
Understanding Angular Overlap Method in Advanced Inorganic Chemistry
Exploring the Angular Overlap Method (AOM) in advanced inorganic chemistry provides a qualitative discussion on the physical rationale behind the theory of complexes. By considering the interaction of atomic orbitals and the degree of overlap, AOM offers insights into energy quantification in coordi
0 views • 15 slides
Understanding Atomic Orbitals and Electron Arrangement
Learn about the properties of atomic orbitals and how they determine the distance from the nucleus and the shape of orbitals. Explore the main energy levels, sublevels, and the arrangement of electrons following the Aufbau Principle within an atom.
4 views • 28 slides
Understanding Alkynes in Organic Chemistry
Alkynes are unsaturated hydrocarbons with at least one triple bond, following a molecular formula of CnH2n-2. This group of compounds is discussed in Chapter three, covering topics like structure, hybridization, common naming, physical properties, preparation, and reactions. The sp hybridization of
1 views • 20 slides
Understanding Atomic Structure and Interatomic Bonding
Atomic structure is defined by the atomic number (Z) and atomic mass (A). Quantum mechanics governs atomic and subatomic particles, introducing discrete energy levels. The Bohr atomic model describes electrons orbiting the nucleus in defined orbitals. Quantum numbers characterize electron properties
0 views • 15 slides
Introduction to Atomic Masses and Mass Spectrometry
Understanding atomic masses, isotopes, and mass spectrometry in the context of chemistry, particularly the concept of standard atomic mass unit (amu) and its application in measuring the masses of atoms. The content delves into the composition of atoms, isotopes, and how to determine the mass of an
1 views • 20 slides
Exploring Physics Beyond the Standard Model through Atomic Spectroscopy
Unlocking the mysteries of physics beyond the Standard Model using atomic spectroscopy techniques. Delving into the shortcomings of the Standard Model and the quest for new physics through precision measurements of atomic forces and isotope shifts in heavy atoms. Highlighting the importance of the p
0 views • 47 slides
Exploring the Fundamentals of Atomic Structure and Matter
Delve into the intricate world of atomic structure and matter through a comprehensive study covering topics such as subatomic particles, atomic models, and key concepts like atomic number and mass. Explore the fascinating properties of atoms, their components, and their interactions, gaining insight
2 views • 15 slides
Understanding the Basic Structure of Atoms: Atomic Theory Class #1
Exploring the fundamental components of atoms, including protons, neutrons, and electrons, their charges, symbols, masses, and locations within the atom. We delve into the concept of atomic mass units (AMU) and the structure of the nucleus, as well as the behavior of electrons in relation to the nuc
0 views • 108 slides
Chemistry Review Exercise: Periodic Table and Atomic Properties Review
This detailed content covers a review exercise on the Periodic Table and Atomic Properties of various elements. It includes information on atomic structures, fundamental physical constants, and common elements found in the Periodic Table. The content explores elements from Hydrogen to Osmium, detail
0 views • 16 slides
The Rutherford Atom and Its Structure
The Rutherford atom is characterized by a thin circle of negative electrons surrounding a tiny positive nucleus. In this model, the electrons are in a diffuse cloud around the nucleus, forming the majority of the atomic volume but only a small fraction of the mass. Protons define an element's atomic
0 views • 17 slides
Understanding Electricity at the Atomic Level
Explore the fascinating world of electricity at the atomic level, delving into concepts such as the movement of electrons, components of an atom, electron orbits, atomic numbers, and the behavior of elements like copper and sulfur. Discover how these fundamental principles shape the properties of ma
0 views • 28 slides
Crystal Field Theory and Color Exhibited by Coordination Compounds
Crystal Field Theory (CFT) explains the colors exhibited by coordination compounds based on the absorption of light and electron transitions in d-orbitals. The theory describes how ligands interact with transition metal ions, causing the d-orbitals to split in energy levels. This split results in th
0 views • 30 slides
Understanding Atomic Mass and Isotopes in Atoms
Explore the concept of atomic mass in atoms, learn to compute atomic mass and mass number, identify isotopes, and calculate the number of neutrons in an atom. Understand the significance of the atomic number and mass number in determining the characteristics of elements.
0 views • 14 slides
Understanding Atomic Structure in Introductory Chemistry
Explore the basics of atomic structure in Introductory Chemistry, covering topics such as representing elements with atomic symbols, isotopes, and atomic bookkeeping. Learn through visual aids and examples to enhance your understanding of elements, isotopes, and atomic properties.
0 views • 21 slides
The Importance of Atomic Clocks in Modern Technology
Explore the significance of precise timekeeping provided by atomic clocks, the fundamentals of atomic clocks, the advancements in single-atom optical clocks by experts like D. J. Wineland from NIST Boulder, the role of atomic energy state superpositions, and the practical operation of atomic clocks.
0 views • 33 slides
Understanding Atomic Orbitals: Counting, Subshells, Energies, and Electrons
Learn about the basics of atomic orbitals, including the counting of orbitals in shells and subshells, the distribution of electrons in different energy levels, and the symmetrical nature of orbital labeling. Dive into the rules governing electron placement based on quantum mechanics and explore the
0 views • 6 slides
Understanding Atomic Structure: The Journey from Ancient Philosophers to Modern Theories
Exploring the evolution of atomic theory from ancient times to John Dalton's contributions, the concept of indivisible atoms forming elements, atomic spectra of hydrogen, and Bohr's atomic model. Delve into the limitations of Bohr's model and the complexities of multi-electron atoms.
0 views • 15 slides
Understanding Atomic Structure and Laws of Matter
Explore the fundamental concepts of atomic structure, including the size of atoms, evidence of atomic composition, and key laws of matter such as the Law of Conservation of Mass. Delve into the historical understanding of atomic structure and the contributions of significant scientists like John Dal
0 views • 29 slides
General Chemistry Homework Questions
Solve quantum number problems, determine total orbitals, electron configurations, isoelectronic series, atomic radius order, and ionization energies in this set of General Chemistry questions.
0 views • 10 slides
Understanding Periodic Trends in Atomic Characteristics
Periodic trends in atomic characteristics such as atomic radius, ionization energy, ion size, and electronegativity are essential in understanding the behavior of elements. These trends are influenced by factors like the energy levels and the number of protons and electrons in an atom. The atomic ra
0 views • 23 slides
Exploring Atomic Dimensions: Scanning Probe Microscopy
Delve into the world of nanoscale imaging with Scanning Probe Microscopy (SPM) techniques like Scanning Tunneling Microscopy (STM) and Atomic Force Microscope (AFM). Unlike optical microscopes, SPM methods break the diffraction limit by relying on intermolecular forces and quantum tunneling for unpa
0 views • 26 slides
Unveiling the Atomic Theory of Matter: Highlights
Matter comprises atoms and molecules in continuous motion, as evidenced by Brownian motion. The precise size of atoms was estimated at 10^-10 meters through experiments. The arrangements of molecules differ in solids, liquids, and gases, with Feynman highlighting the significance of the atomic hypot
0 views • 33 slides