Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Terminal Base, Air Terminal Base, Lightning Rod Terminal Base, Lightning Rod Bas
Nexus Metal & Alloys: We are Manufacturer, Supplier and Exporter of Terminal Base, Air Terminal Base, Lightning Rod Terminal Base, Lightning Rod Base, Electrical Terminals Base, Solid Terminal Base, Black Terminal Base etc from Khetwadi, Maharashtra, India. We Export to Worldwide.
3 views • 3 slides
Cannizzaro Reaction: Separation of Benzoic Acid and Benzyl Alcohol
The Cannizzaro reaction involves the separation of benzoic acid and benzyl alcohol by utilizing various reagents and procedures such as dissolving potassium benzoate, washing with ether, and treatment with different solutions to isolate the desired compounds. The process involves multiple steps incl
7 views • 4 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Acid-Base Balance in Health and Disease
Many critical illnesses can disrupt acid-base balance, indicating underlying diseases or organ damage. Interpretation of disturbances requires analyzing arterial blood gases, plasma electrolytes, and compensatory mechanisms. Acid-base disorders are classified into respiratory acidosis, respiratory a
3 views • 26 slides
Understanding Acid-Base Balance in the Body: Importance and Regulation
Acid-base balance is crucial for maintaining optimal health, as slight deviations in hydrogen ion concentration can impact enzyme activity and metabolic processes. The body employs various defense mechanisms to regulate pH levels, involving buffers, lungs, and kidneys. Strong acids release more H+ i
3 views • 48 slides
Industrial Production of Citric Acid: Fermentation Processes and Applications
Citric acid is industrially produced through microbial fermentation processes. It is widely used in various industries such as food, pharmaceuticals, and beverage production. Different methods like surface culture fermentation are employed for citric acid manufacture, with fungi like Aspergillus nig
4 views • 18 slides
Limit Test of Iron Based on Color Reaction with Thioglycollic Acid
The limit test for iron involves the reaction of iron in ammoniacal solution with citric acid and thioglycollic acid to form a reddish-purple color. By comparing the color produced with a standard solution, the presence of iron is determined. Citric acid prevents precipitation of iron, while thiogly
1 views • 5 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Overview of Ammonium Chloride: Properties, Preparation, and Uses
Ammonium Chloride, with the formula NH4Cl, is a compound containing not less than 99.5% NH4Cl. It is prepared commercially by neutralizing ammonia with hydrochloric acid or treating ammoniacal gas liquors with lime. The compound is essential for maintaining acid-base equilibrium, acts as an expector
2 views • 4 slides
Determination of Acetic Acid Content in Vinegar Experiment
The experiment aims to measure the total acid concentration in a specific brand of vinegar through a titration process using NaOH solution. The procedure involves titrating the vinegar solution with NaOH until a pink color appears, calculating the concentration of acetic acid, and determining the pe
3 views • 8 slides
Understanding Enzyme Catalysis and Active Site Role
Enzymes play a crucial role in catalyzing biochemical reactions by stabilizing transition states through their active sites. Different mechanisms like acid-base, covalent, metal, and electrostatic interactions are employed for stabilization. Acid-base catalysis involves acceleration without being co
1 views • 21 slides
Understanding Acid-Base Disturbances in Medical Practice
Explore the intricacies of acid-base physiology, mechanisms, and interpretation, along with the life-saving roles of blood buffers, respiratory reactions, and renal mechanisms. Learn how to handle acid loads and interpret abnormalities confidently, guided by expert insights.
1 views • 29 slides
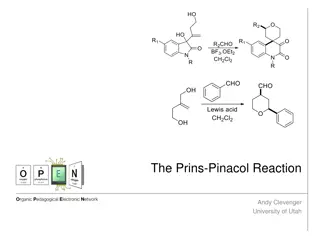
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Synthesis of Salicylic Acid: Theory, Derivatives, and Applications
Salicylic acid is synthesized from methyl salicylate through ester hydrolysis with aqueous alkali. It is a versatile compound used in organic synthesis, as a plant hormone, and derived from salicin metabolism. The derivatives of salicylic acid can minimize gastric disturbances and enhance therapeuti
4 views • 12 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of an unknown acid or base solution. By neutralizing the solution with the opposite component and reaching the equivalence point, students can calculate the concentration using stoichiometry principles. This method d
1 views • 24 slides
Overview of Salicylic Acid and its Derivatives
Salicylic acid is known for its antiseptic, germicidal, and analgesic properties, used in various applications such as preservatives, wart treatments, and pain relief. Different derivatives of salicylic acid are developed to minimize side effects and enhance efficacy. Acetyl salicylic acid (ASA), co
0 views • 15 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
Understanding Acid-Base Disorders in Critical Care
Explore the intricate details of acid-base disorders, including metabolic acidosis with anion gap, high and normal anion gap metabolic acidosis, causes of lactic acidosis, and important considerations like hypoalbuminemia. Learn about the delta ratio and how to interpret it in the context of acid-ba
0 views • 31 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of unknown acids or bases. Through neutralization reactions, the concentration of a solution can be calculated based on the stoichiometry of the reaction. This method dates back to the late 18th century and is crucia
0 views • 24 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
Assay of Aspirin by Indirect Acid-Base Titration
Indirect titration, also known as residual titration, is an analytical technique used to determine the weight of an unknown sample by employing excess standard solution. In the case of aspirin, which is a weak acid undergoing slow hydrolysis, back titration with NaOH and HCl is utilized to overcome
1 views • 13 slides
Understanding Acid-Base Balance in Patients
Explore the assessment and care of patients with acid-base imbalances, including the importance of normal blood pH levels, the role of acids and bases in body chemistry, and the calculation of free hydrogen ion levels. Discover how acid-base balance is maintained and the critical impact of pH change
0 views • 41 slides
Understanding Purine Degradation and Gout
Purine degradation pathway involves the breakdown of dietary nucleic acids, mainly from meat, into uric acid through specific enzymatic steps. Excessive uric acid production can lead to conditions like gout and hyperuricemia. Humans excrete uric acid in the urine as the final product, while other an
1 views • 12 slides
Understanding Acid-Base Physiology in the Human Body
Acid-Base homeostasis plays a crucial role in maintaining the optimal acidity of body fluids through a balance of chemical buffers and physiological processes. Disruptions in this balance can lead to various effects on the central nervous system and ionized calcium levels. Understanding the concepts
0 views • 21 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Understanding Acid-Base Balance and Regulation in the Body
Acid-base balance is crucial for maintaining optimal physiological functions. This content explains the definitions of acids and bases, differentiates between strong and weak varieties, explores the significance of pH control in body fluids, and delves into the roles of buffers, kidneys, and lungs i
0 views • 20 slides
Understanding Salt Hydrolysis in Chemistry
Salt hydrolysis is a chemical process in which a salt reacts with water to produce an acid and a base. This reaction occurs when the cation or anion of the salt interacts with water, resulting in either an acidic or basic solution. The type of hydrolysis depends on the strength of the acid and base
0 views • 6 slides
Understanding Acidosis and Alkalosis in Acid-Base Disorders
Acid-base disorders, involving acidosis and alkalosis, are common in clinical practice. These conditions are characterized by changes in pH levels, with acidemia (pH < 7.35) and alkalemia (pH > 7.45) representing acidosis and alkalosis, respectively. The primary causes of these imbalances lie within
0 views • 17 slides
Acid-Base Titration Lab Procedure & Calculations
Conduct an acid-base titration experiment to determine the molarity of an unknown NaOH solution using HCl as the titrant. Follow the outlined steps, record data, perform calculations, and analyze the results to understand the principles of this chemical reaction. Learn about indicators, mole ratios,
0 views • 7 slides
Common Organic Acids and Their Uses in Various Industries
Citric acid, lactic acid, salicylic acid, and tartaric acid are natural acids found in various fruits. They have diverse applications in food and beverage, pharmaceutical, and cosmetic industries. These acids are used for flavoring, cleaning, as food additives, and in the preparation of various prod
0 views • 4 slides
Equilibrium and Acid-Base Problems in Chemistry Lecture
In this lecture, topics such as Advanced Equilibrium, Acid/Base Equilibria, Systematic Method for solving chemical problems, Strong Acid/Strong Base scenarios, and General Comments on reactions are discussed. Examples using the systematic method are provided for practical understanding. Key points o
0 views • 13 slides
Synthesis of Banana Oil in Chemistry Class
The synthesis of banana oil involves a reaction between carboxylic acid and alcohol catalyzed by H2SO4 to produce isoamyl acetate. The mechanism includes nucleophilic acyl substitution with two main reaction steps - addition and elimination. The process shifts the equilibrium to favor product format
0 views • 16 slides
Understanding Serum Uric Acid Levels and Hyperuricemia
Serum uric acid is a crucial marker of purine metabolism, with elevated levels indicating hyperuricemia which can lead to conditions like Gout. The measurement of serum uric acid helps in diagnosing hyperuricemia, with serum being the preferred specimen for testing. Various factors such as diet, gen
0 views • 12 slides
Understanding Fatty Acid Metabolism in Animals
Animals cannot convert fatty acids into glucose due to the inability to synthesize glucose from fatty acids. The process involves acetyl-CoA not being converted into pyruvate or oxaloacetate, leading to the citric acid cycle and differences between fatty acid synthesis and degradation pathways. Key
0 views • 8 slides
Understanding Acid-Base Disorders and Blood Gas Analysis
This educational content provides definitions, formulas, and explanations related to acid-base disorders, blood gas analysis, dissociation constants, the Henderson-Hasselbalch equation, and carbonic acid in clinical pathology. Learn about acids, bases, pH, ionization constants, and how these concept
0 views • 35 slides
Understanding Acid-Base Chemistry Principles
Acid-base chemistry involves the autoionization of water, pH calculations using the ion product constant for water (Kw), and the categorization of solutions as neutral, acidic, or basic based on the concentrations of hydronium and hydroxide ions. Definitions of acids and bases, conjugate acid-base p
0 views • 31 slides