Chemical equilibrium
Chemical equilibrium is a state where reactants and products reach a balance in a reaction. Learn about reversible and irreversible reactions, equilibrium constants, and how they affect chemical analysis. Discover the accuracy and applications of volumetric analysis, including titration methods like
3 views • 13 slides
Terminal Base, Air Terminal Base, Lightning Rod Terminal Base, Lightning Rod Bas
Nexus Metal & Alloys: We are Manufacturer, Supplier and Exporter of Terminal Base, Air Terminal Base, Lightning Rod Terminal Base, Lightning Rod Base, Electrical Terminals Base, Solid Terminal Base, Black Terminal Base etc from Khetwadi, Maharashtra, India. We Export to Worldwide.
3 views • 3 slides
Understanding Acid-Base Balance in Health and Disease
Many critical illnesses can disrupt acid-base balance, indicating underlying diseases or organ damage. Interpretation of disturbances requires analyzing arterial blood gases, plasma electrolytes, and compensatory mechanisms. Acid-base disorders are classified into respiratory acidosis, respiratory a
3 views • 26 slides
Understanding Acid-Base Balance in the Body: Importance and Regulation
Acid-base balance is crucial for maintaining optimal health, as slight deviations in hydrogen ion concentration can impact enzyme activity and metabolic processes. The body employs various defense mechanisms to regulate pH levels, involving buffers, lungs, and kidneys. Strong acids release more H+ i
3 views • 48 slides
Industrial Production of Citric Acid: Fermentation Processes and Applications
Citric acid is industrially produced through microbial fermentation processes. It is widely used in various industries such as food, pharmaceuticals, and beverage production. Different methods like surface culture fermentation are employed for citric acid manufacture, with fungi like Aspergillus nig
4 views • 18 slides
Understanding Equilibrium for Moving Objects
Objects can be in static equilibrium when at rest or dynamic equilibrium when moving at a constant speed. Equilibrium is maintained when there is no net force to change the state of motion. This equilibrium is possible when forces either cancel out or there is no force acting on the object. Friction
0 views • 8 slides
Limit Test of Iron Based on Color Reaction with Thioglycollic Acid
The limit test for iron involves the reaction of iron in ammoniacal solution with citric acid and thioglycollic acid to form a reddish-purple color. By comparing the color produced with a standard solution, the presence of iron is determined. Citric acid prevents precipitation of iron, while thiogly
1 views • 5 slides
Understanding Chemical Equilibrium in Reversible Reactions and Laws
Chemical equilibrium in reversible reactions involves the balance between forward and backward reactions, as governed by laws like the law of mass action and the law of chemical equilibrium. These laws help in understanding the rates of reactions, equilibrium constants, and the relationship between
1 views • 12 slides
Understanding Dynamic Equilibrium in Chemical Reactions
Explanation of reversible reactions, dynamic equilibrium, and the characteristics of equilibrium in chemical systems. Covers the concept of reversible reactions, dynamic equilibrium, rules for dynamic equilibrium, and examples to illustrate these concepts visually.
0 views • 54 slides
Understanding Le Chatelier's Principle in Chemical Equilibrium
Le Chatelier's Principle states that when a system at equilibrium is disturbed by changes in concentration, temperature, or pressure, the equilibrium shifts to counteract the change. This principle can be applied to predict the direction of equilibrium when changes occur. Changes in concentration, p
0 views • 10 slides
Understanding Reaction Isotherms and Equilibrium Constants
Explore reaction isotherms and equilibrium constants through Vant Hoff's and Gibbs free energy equations. Learn about the relationship between Gibbs free energy, equilibrium constant, and temperature dependence. Discover how these concepts are applied in determining the direction of chemical reactio
1 views • 18 slides
Overview of Ammonium Chloride: Properties, Preparation, and Uses
Ammonium Chloride, with the formula NH4Cl, is a compound containing not less than 99.5% NH4Cl. It is prepared commercially by neutralizing ammonia with hydrochloric acid or treating ammoniacal gas liquors with lime. The compound is essential for maintaining acid-base equilibrium, acts as an expector
2 views • 4 slides
Determination of Acetic Acid Content in Vinegar Experiment
The experiment aims to measure the total acid concentration in a specific brand of vinegar through a titration process using NaOH solution. The procedure involves titrating the vinegar solution with NaOH until a pink color appears, calculating the concentration of acetic acid, and determining the pe
2 views • 8 slides
Understanding Enzyme Catalysis and Active Site Role
Enzymes play a crucial role in catalyzing biochemical reactions by stabilizing transition states through their active sites. Different mechanisms like acid-base, covalent, metal, and electrostatic interactions are employed for stabilization. Acid-base catalysis involves acceleration without being co
1 views • 21 slides
Synthesis of Salicylic Acid: Theory, Derivatives, and Applications
Salicylic acid is synthesized from methyl salicylate through ester hydrolysis with aqueous alkali. It is a versatile compound used in organic synthesis, as a plant hormone, and derived from salicin metabolism. The derivatives of salicylic acid can minimize gastric disturbances and enhance therapeuti
4 views • 12 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of an unknown acid or base solution. By neutralizing the solution with the opposite component and reaching the equivalence point, students can calculate the concentration using stoichiometry principles. This method d
1 views • 24 slides
Overview of Salicylic Acid and its Derivatives
Salicylic acid is known for its antiseptic, germicidal, and analgesic properties, used in various applications such as preservatives, wart treatments, and pain relief. Different derivatives of salicylic acid are developed to minimize side effects and enhance efficacy. Acetyl salicylic acid (ASA), co
0 views • 15 slides
Equilibrium of Rigid Bodies: Moments, Couples, and Forces
The topic covers the equilibrium of rigid bodies with a focus on moments, couples, and forces. It discusses concepts such as moments of a force, Varignon's theorem, types of supports, and equilibrium in two and three dimensions. The equilibrium in two-force members and three-force members is explain
0 views • 23 slides
Understanding Acid-Base Disorders in Critical Care
Explore the intricate details of acid-base disorders, including metabolic acidosis with anion gap, high and normal anion gap metabolic acidosis, causes of lactic acidosis, and important considerations like hypoalbuminemia. Learn about the delta ratio and how to interpret it in the context of acid-ba
0 views • 31 slides
Understanding Consumer Equilibrium in Economics
Consumer equilibrium refers to the point where a consumer maximizes satisfaction by spending income on commodities. In single commodity case, equilibrium is achieved when marginal utility equals price. For two commodities, equilibrium is reached when the ratio of marginal utility to price is equal.
0 views • 7 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Understanding Chemical Equilibrium in Reversible Reactions
Chemical equilibrium occurs when the concentrations of reactants and products remain constant over time in a reversible reaction. Reaction rate is proportional to concentration, and equilibrium is reached when the rate of formation equals the rate of consumption in both directions. Reversible reacti
0 views • 25 slides
Understanding Acid-Base Balance in Patients
Explore the assessment and care of patients with acid-base imbalances, including the importance of normal blood pH levels, the role of acids and bases in body chemistry, and the calculation of free hydrogen ion levels. Discover how acid-base balance is maintained and the critical impact of pH change
0 views • 41 slides
Understanding Acid-Base Physiology in the Human Body
Acid-Base homeostasis plays a crucial role in maintaining the optimal acidity of body fluids through a balance of chemical buffers and physiological processes. Disruptions in this balance can lead to various effects on the central nervous system and ionized calcium levels. Understanding the concepts
0 views • 21 slides
Understanding Acid-Base Balance and Regulation in the Body
Acid-base balance is crucial for maintaining optimal physiological functions. This content explains the definitions of acids and bases, differentiates between strong and weak varieties, explores the significance of pH control in body fluids, and delves into the roles of buffers, kidneys, and lungs i
0 views • 20 slides
Insights into Persuasion and Equilibrium in Multidimensional Cheap Talk
Explore the dynamics of multidimensional cheap talk, focusing on sender-receiver interactions, influential equilibrium, welfare rankings, and fragility to asymmetries. Lessons touch on bubbling equilibrium, influential equilibrium issues, welfare rankings preferences, and the impact of asymmetric pr
0 views • 20 slides
Understanding Equilibria in Populations: Hardy-Weinberg Principle
Exploring the concept of equilibria in populations, focusing on Hardy-Weinberg principles and its implications. The discussion covers allele distributions, genotype frequencies, maintenance of equilibrium across generations, and scenarios where equilibrium may be violated. Key points include basic p
0 views • 58 slides
Understanding Groundwater Sustainability and Equilibrium Dynamics
This content delves into the concepts of groundwater sustainability, equilibrium, and capture in relation to groundwater management. It explores transitional storage, groundwater mining, and debunks the water budget myth. The images and explanations illustrate how pumping affects aquifers, the evolu
0 views • 11 slides
Understanding Acidosis and Alkalosis in Acid-Base Disorders
Acid-base disorders, involving acidosis and alkalosis, are common in clinical practice. These conditions are characterized by changes in pH levels, with acidemia (pH < 7.35) and alkalemia (pH > 7.45) representing acidosis and alkalosis, respectively. The primary causes of these imbalances lie within
0 views • 17 slides
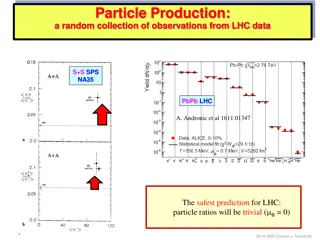
Insights into Particle Ratios and Equilibrium Dynamics at LHC
Collection of observations from LHC data regarding particle ratios and the successful Thermal Model. Discusses the concept of equilibrium, onset of equilibrium, relationship to QGP phase, and potential solutions through out-of-equilibrium studies. Also delves into size/volume dependence, strangeness
0 views • 11 slides
Understanding Homogenous Chemical Equilibrium
Homogenous chemical equilibrium occurs when reactants and products are in the same phase. This equilibrium remains independent of the volume of the reaction mixture. The concept is illustrated through the example of the Hydrogen-Iodide system and a generic reaction A + B --> 2C. Partial pressure pla
0 views • 56 slides
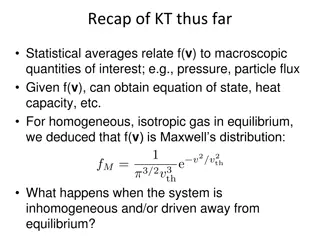
Insights into Non-equilibrium Kinetic Theory: Inhomogeneous Systems
Statistical averages in kinetic theory connect distribution functions to macroscopic properties like pressure and particle flux. When systems are inhomogeneous or away from equilibrium, local equilibrium breaks down, leading to slow relaxation processes towards global equilibrium. The evolution of p
0 views • 12 slides
Understanding Equilibriums in Physics
Equilibrium in physics refers to the state of a body where there is no change in translational or rotational motion. This state can be classified into static equilibrium (when total force and torque are zero) and dynamic equilibrium (when a body is in uniform motion with zero resultant force and tor
0 views • 9 slides
Understanding Market Equilibrium
Market equilibrium is achieved when the quantity demanded equals the quantity supplied at a specific price, ensuring a balance in the marketplace. Demand and supply schedules play a crucial role in determining market equilibrium, with excess supply or demand occurring when prices deviate from the eq
0 views • 12 slides
Understanding Game Abstraction and Equilibrium
Extensive-Form Game Abstraction with Bounds delves into the complexities of game abstraction, exploring theoretical guarantees, algorithmic challenges, and equilibrium-finding processes. The difficulty of game abstraction is examined, highlighting issues such as pathologies and the struggle to optim
0 views • 22 slides
Understanding Chemical Equilibrium in Chemistry
Exploring the concept of chemical equilibrium in chemistry, where reactions can occur in both forward and reverse directions to an appreciable extent. Learn about basic equilibrium principles, equilibrium problems, manipulating reactions, and common example problems in nomenclature. Understand how r
0 views • 24 slides
Equilibrium and Acid-Base Problems in Chemistry Lecture
In this lecture, topics such as Advanced Equilibrium, Acid/Base Equilibria, Systematic Method for solving chemical problems, Strong Acid/Strong Base scenarios, and General Comments on reactions are discussed. Examples using the systematic method are provided for practical understanding. Key points o
0 views • 13 slides
Understanding Serum Uric Acid Levels and Hyperuricemia
Serum uric acid is a crucial marker of purine metabolism, with elevated levels indicating hyperuricemia which can lead to conditions like Gout. The measurement of serum uric acid helps in diagnosing hyperuricemia, with serum being the preferred specimen for testing. Various factors such as diet, gen
0 views • 12 slides
Understanding Fatty Acid Metabolism in Animals
Animals cannot convert fatty acids into glucose due to the inability to synthesize glucose from fatty acids. The process involves acetyl-CoA not being converted into pyruvate or oxaloacetate, leading to the citric acid cycle and differences between fatty acid synthesis and degradation pathways. Key
0 views • 8 slides
Understanding Acid-Base Chemistry Principles
Acid-base chemistry involves the autoionization of water, pH calculations using the ion product constant for water (Kw), and the categorization of solutions as neutral, acidic, or basic based on the concentrations of hydronium and hydroxide ions. Definitions of acids and bases, conjugate acid-base p
0 views • 31 slides