Calculating Limiting Reagent in Chemical Reactions
Calculating the amount of reactants in excess and the limiting reagent plays a crucial role in determining the maximum extent of a chemical reaction. By using the relative numbers of moles of substances as shown in balanced equations, one can identify the reactant that is fully utilized, hence limiting the reaction. This process helps in predicting the amount of products formed accurately. Explore examples and worked problems involving limiting reagents to enhance your understanding of stoichiometry.
Uploaded on Sep 12, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Starter Calculate the mass of hydrogen required to react completely with 56g of nitrogen. N2(g) + 3H2(g) 2NH3(g)
Learning Intention Learn how to calculate how much of a particular reactant is in excess from the balanced equation. By the end of today you should be able to: Determine which reactant is the limiting reagent
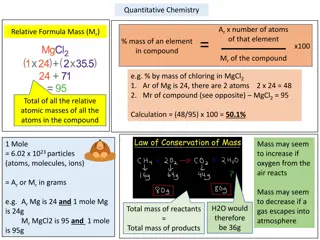
Limiting agent You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reaction. Other reactants are said to be in excess.
Calculations involving limiting reagents As soon as one of the reactant in a chemical reaction is used up the reaction stops. Any other reactant which is left over is said to be in excess . The reactant which is used up determines the mass of product formed. You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reaction. Other reactants are said to be in excess.
Calculations involving limiting reagent Worked example. Which reactant is in excess when 10g of calcium carbonate reacts with 100cm3 of 1 mol l-1 hydrochloric acid? HCl limiting reagent
Calculations involving limiting reagent Worked example 2. 1.2g of magnesium was added to 100cm3 2 mol l-1 hydrochloric acid. Calculate the reagent in excess? Magnesium limiting reagent
Calculations involving limiting reagent Examples. For each of the following reactions calculate which reagent is the limiting reagent? a) 4.86g magnesium added to 250cm3 2 mol l-1 hydrochloric acid Mg b) 2.7g aluminium added to 200cm3 1 mol l-1 hydrochloric acid HCl c) 2.43g magnesium added to 200cm3 1 mol l-1 sulphuric acid Mg d) 3.27g zinc added to 100cm3 0.2 mol l-1 hydrochloric acid. HCl
A 50.6 g sample of Mg(OH)2 is reacted with 45.0 g of HCl. What mass of magnesium chloride will be produced? Mg(OH)2 + 2 HCl MgCl2 + 2 H2O a) How many moles of Mg(OH)2 are there in 50.6g? b) How many moles of HCl are there in 45g? c) How many moles of Mg(OH)2 would react with that amount of moles of HCl? d) Which is the limiting reagent? e) How many moles of MgCl2 will be produced from this reaction? f) How many grams of MgCl2 will be produced from this reaction?
2. a) Calculate which reagent is in excess when 3.27g of zinc is reacted with 100cm3 of 2.0 mol l-1 hydrochloric acid. b) What mass of hydrogen gas will be produced? 0.1 g
Calculations for you to try. 1. What mass of calcium oxide is formed when 0.4 g of calcium reacts with 0.05 mole of oxygen? 2Ca + O2 2CaO 0.56g
2. What mass of hydrogen is formed when 3.27g of zinc is reacted with 25cm3 of 2 mol l-1 hydrochloric acid? Zn + 2HCl ZnCl2 + H2 0.05 g
Vitamin C is in excess, therefore iodine would have been decolourised.