Understanding Downstream Processing in Bioprocessing
Downstream processing plays a crucial role in bioprocessing after fermentation, focusing on separating, purifying, and packaging the final product. It involves stages like cell disruption, removal of insoluble particles, product recovery, and purification methods such as filtration and centrifugation. Various techniques like mechanical and non-mechanical processes are used for cell disruption to release intracellular products. The overall goal is to obtain a highly purified end product ready for the market.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
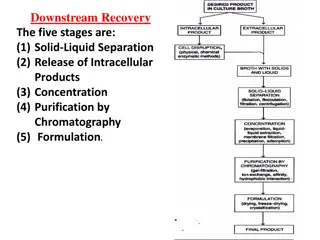
Chapter 8 Downstream processing
Outline Introduction Cell disruption Removal of insoluble Product isolation Product purification Product polishing
Typical Bioprocessing Stock Culture Raw Materials Medium Formulation Shake Flasks Sterilization Seed fermenter Air Agitator Recovery Purification Products
Downstream processing The various stages of processing that occur after the completion of the fermentation or bioconversion stage, including separation, purification, and packaging of the product. is any treatment of culture broth after fermentation to concentrate and purify the products
Stage in Downstream processing Crude product Product recovery Removal of insoluble Cell disruption Purify the product Finished product market
Cell disruption Some product are intercellular including many enzymes and products required to disrupt the cells and release these products e.g. Yeast Cell disruption can be achieved by both mechanical and non mechanical methods Mechanical sonication and liquid shear homogenization etc. Non mechanical autolysis, osmosis shock etc.
Removal of Insoluble Separation of cells, cell debris or other particulate matter from fermentation broth culture Typical operations to achieve this: 1) Filtration 2) Centrifugation 3) Sedimentation 4) Flocculation a process where a solute comes out of solution in the form of floc or flakes. 5) Gravity settling
Filtration A mechanical operation used for the separation of solids from fluids (liquids or gases) by interposing a medium to porous membrane through which the fluid can pass, but the solids in the fluid are retained.
Filtration Separation of particles from liquid by applying a pressure to the solution to force the solution through a filter. Filter are materials with pores Particles larger than the pore size of the filter are retained by filter. Particles smaller than the pore size of the filter pass through the filter along with the liquid
Filtration The solid particles deposited on the filter form a layer, which is known as filter cake. All the solid particles from the feed are stopped by the cake ,and the cake grows at the rate at which particles are bought to its surface. All of the fluid goes through the cake and filter medium.
Continuous Rotary Vacuum filter It is one of the most commonly used type of filter in fermentation. The drum is pre coated prior to filtration. A small agent of coagulating is added to the broth before it is pumped into the filter. The drum rotates under vacuum and a thin layer of cells sticks to the drum. The thickness of the layer increases in the section designed for forming the cake.
Continuous Rotary filter Liquid filtrate Filter Hollow spokes Cell mass Perforate d drum Knife
Points to be considered while selecting the filter medium: Ability to build the solid. Minimum resistance to flow the filtrate. Resistance to chemical attack. Minimum cost. Long life
Centrifugation Centrifugation is used to separate particles of 100 0.1 micrometer from liquid by gravitational forces. It depends on particles size, density difference between the cells and the broth and broth viscosity. Use of the centrifugal force for the separation of mixtures More-dense components migrate away from the axis of the centrifuge Less-dense components migrate towards the axis. Types of centrifuges used are Tubular bowl centrifuge, multi-chamber centrifuge, disc bowl centrifuge etc.
Sedimentation It is applicable only for large particles greater than 100 micrometer flocs. It is a slow process and takes ~3 hours. It is used in process like activated sludge effluent treatment. It s a free settling process depends only on gravity.
Flocculation Process where a solute comes out of solution in the form of flocs or flakes. Particles finer than 0.1 m in water remain continuously in motion due to electrostatic charge which causes them to repel each other Once their electrostatic charge is neutralized (use of coagulant) the finer particles start to collide and combine together . These larger and heavier particles are called flocs.
Product Isolation Removal of those components whose properties vary markedly from that of the desired product. Water is the chief impurity a) Isolation steps are designed to remove it (i.e.dialysis) b) Reducing the volume c) Concentrating the product. d) Liquid liquid extraction, adsorption, ultrafiltration, and precipitation are some of the unit operations involved.
Liquid -Liquid extraction It is a separation process that takes the advantage of the relative solubilities of solute in immiscible solvents. Solute is dissolved more readily and becomes more concentrated in the solvent in which it has a higher solubility. A partial separation occurs when a number of solutes have different relative solubilities in the two solvents used. Solvent should be non toxic, selective, inexpensive and immiscible with broth and should have a high distribution coefficient for the product.
Adsorption is a surface phenomenon It is the binding of molecules to the surface and different from absorption. The binding to the surface is weak and reversible. Compounds containing chromogenic group are usually strongly adsorbed on activated carbon. Common adsorbent used are activated carbon, silica gel ,alumina becoz they present enormous surface areas per unit weight.
Ultrafiltration UF is basically a pressure-driven separation process. The operating pressure is usually between 0.1 and 1 MPa.
Ultrafiltration UF is governed by a screening principle and dependent on particle size. UF membranes have a pore size between 1 nm and 100 nm (10 and 2000 ), thus allowing retention of compounds with a molecular weight of 300 to 500 000 Dalton. Typically, the process is suitable for retaining biomolecules, bacteria, viruses, polymers, colloidal particles and sugar molecules.
Precipitation Formation of a solid in a solution during a chemical reaction. Solid formed is called the precipitate and the liquid remaining above the solid is called the supernate.
Precipitation Salts such as ammonium & sodium sulphate are used for proteins to precipitate. Organic solvents methanol used to precipitate dextrans. Chilled ethanol and acetone used for protein precipitation. Non ionic polymer such as polyethylene glycol used in precipitation.
Product Purification Done to separate those contaminants that resemble the product very closely in physical and chemical properties. Expensive to carry out Require sensitive and sophisticated equipment Significant fraction of the entire downstream processing expenditure. Examples of operations include affinity, size exclusion, reversed phase chromatography, crystallization and fractional precipitation.
Product purification Removing components with properties similar to those of the products Chromatography Separates molecules by their chemical and physical difference Adsorption chromatography Affinity chromatography Ion exchange chromatography e.g.
Components - Solute: molecule of interest - Mobile phase( solvent, gas)MF - Stationary phase( solid, liquid)SF Principle - The solute partion between the two phase - The interaction with the solute phase determines how fast the solute migrate in the mobile phase - Solute that interact differently with the stationary phase can be separated
Liquid Chromatography Mobile phase is a liquid. Carried out either in a column or a plane. HPLC In the HPLC technique, the sample is forced through a column that is packed with irregularly or spherically shaped particles or a porous monolithic layer (stationary phase) by a liquid (mobile phase) at high pressure.
Product Polishing Final processing steps which end with packaging of the product in a form that is stable and easily transportable Crystallization, concentration and drying are typical unit operations
Crystallization process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. chemical solid-liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs.
Crystallization process Generation of supersaturation: driving force Nucleation: birth of Liquid mixer solid phase Final product Crystal growth
Crystallization By cooling saturated solution
Drying It involves the transfer of heat to the wet material and the removal of the moisture as water vapor Drying Methods 1 Atmospheric drying 2 Vacuum drying (Decompression ) 3 Freeze-drying(lyophilization) 4 Spray drying
lyophilization Freeze drying Process in which water is removed from a product after it is frozen. It is placed under vacuum allowing the ice to change directly from the solid to vapor without passing through a liquid phase