Investigating the Effect of Salt or Sugar Solutions on Plant Tissue Mass through Osmosis
Explore the impact of different concentrations of salt or sugar solutions on plant tissue mass using the osmosis process with potato cylinders. The experiment involves measuring changes in length and mass after immersing the potato samples in various solutions. Learn about reliability, reproducibility, and ways to enhance the experiment's accuracy and consistency.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
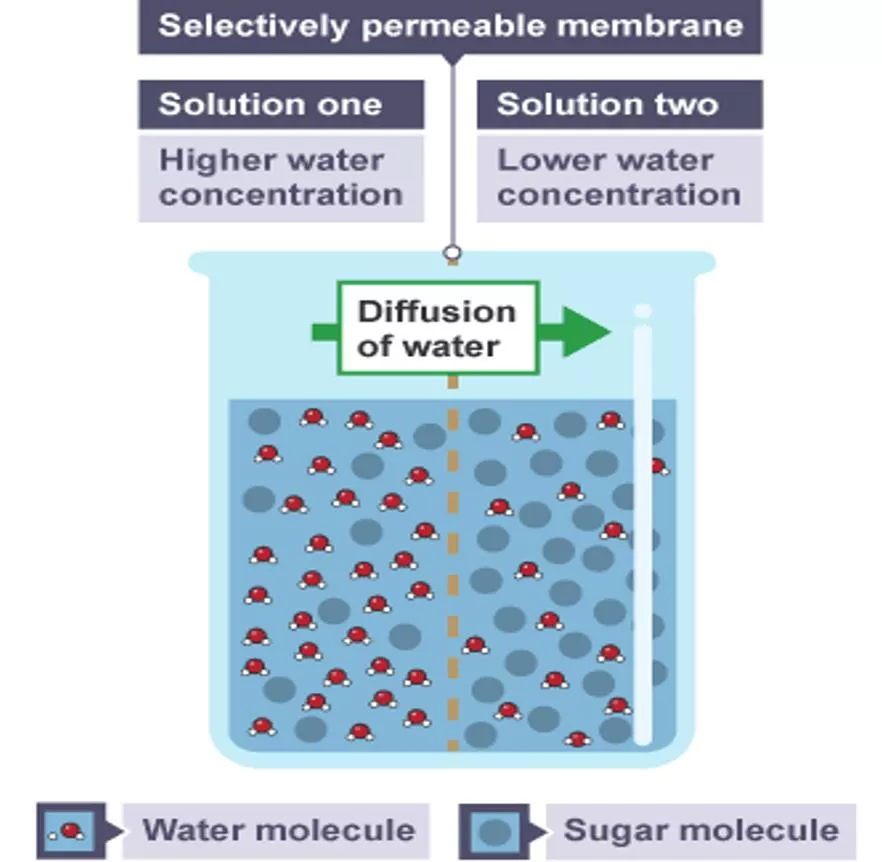
Osmosis Required Practical Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue. Osmosis is the movement of water through a selectively permeable membrane. The water moves from an area of high concentration of water to an area of lower concentration of water. Plant tissues can be used to investigate osmosis. This experiment uses potato. Potato tissue is cut into equal sized cylinders. The potato tissue is left overnight in sugar solution and distilled water. The changes in length and mass can then be accurately compared.
Osmosis Required Practical Osmosis is the diffusion of water molecules, from a region of higher concentration to a region of lower concentration, through a partially permeable membrane. A dilute solution contains a high concentration of water molecules, while a concentrated solution contains a low concentration of water molecules. Partially permeable membranes are also called selectively permeable membranes or semi- permeable membranes. They let some substances pass through them, but not others.
Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue. Osmosis Required Practical METHOD: 1. Use a cork borer to cut three potato cylinders of the same diameter. 2. Trim the cylinders so that they are all the same length (about 3 cm). 3. Accurately measure and record the length and mass of each cylinder. 4. Measure 10 cm3 of the 1.0 M sugar solution and put into the first boiling tube. Label boiling tube as: 1.0 M sugar. 5. Measure 10 cm3 of 0.5 M sugar solution and put into the second boiling tube. Label boiling tube as: 0.5 M sugar. 6. Measure 10 cm3 of the distilled water and put into the third boiling tube. Label boiling tube as water. 7. Add one potato cylinder to each boiling tube. Make sure you know the length and mass of each potato cylinder in each boiling tube. 8. Leave the potato cylinders in the boiling tubes for 30 mins. 9. Remove the cylinders from the boiling tubes and carefully blot them dry with the paper towels. 10. Re-measure the length and mass of each cylinder and calculate the changes.
Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue. Osmosis Required Practical 1.0 M sugar solution 0.5 M sugar solution Distilled water Initial length (mm) Final length (mm) Change in length (mm) Initial mass (g) Final mass in (g) Change in mass in (g)
Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue. Reliability & Reproducibility Reliability: the degree to which the result of a measurement, calculation, or specification can be depended on to be accurate. Task 1. How do we know if results are reliable? 2. How do we know if results are reproducible? 3. How could we improve our method to make it more reliable & reproducible? Reproducibility : The ability of an entire experiment or study to be duplicated, either by the same researcher or by someone else working independently. Reproducing an experiment is called replicating it.
Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue. Osmosis Required Practical Things to remember when answering 6 mark exam questions: 1.Try and remember everything you can about what the question is asking before you start answering it 2.Make at least 6 points 3.Write in full sentences starting with capital letters and ending with full stops 4.Try and answer the question in around five minutes 5.Check your answer to make sure you have not left anything out 6.Remember to use key words when appropriate