Evolution of Atomic Models: From Rutherford to Quantum Mechanics
Various atomic models have been proposed throughout history, starting with John Dalton's idea of atoms as tiny particles to J.J. Thomson's Plum Pudding model. Ernest Rutherford's discovery of the nucleus and Niels Bohr's quantum model of the atom revolutionized our understanding. Bohr's proposal of electrons in fixed energy orbits around the nucleus led to the development of the Quantum Mechanical Model. This model, based on quantum mechanics, provides a deeper insight into the behavior of electrons within an atom, explaining phenomena like energy levels and electron transitions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Quantum Mechanical Model of the Atom Ochran 2014
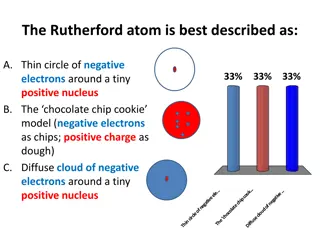
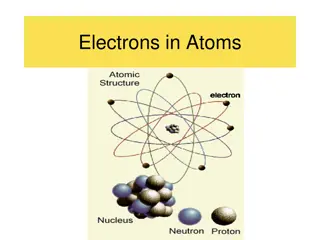
Models of the Atom Rutherford used existing ideas about the atom and proposed an atomic model in which the electrons move around the nucleus, like the planets move around the sun.
Rutherfords atomic model could not explain the chemical properties of elements. Rutherford s model fails to explain why objects change color when heated.
Models of The Atom 1863- John Dalton pictures atoms as tiny, indestructible particles, with no internal structure.
Models of the Atom 1897- J.J. Thomson, a British scientist, discovers the electron. The later leads to his Plum Pudding model. He pictures electrons embedded in a sphere of positive electrical charge.
Models Of the Atom 1911- Ernest Rutherford finds that an atom has a small, dense, positively charged nucleus. Electrons move around the nucleus.
Models of the Atom 1913- Neils Bohr s model, the electron moves in a circular orbit at fixed distances from the nucleus.
The Bohr Model Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. Each orbit has a fixed energy. These fixed energies an electron can have are called energy levels.
These ladder rungs are somewhat like energy levels. The higher an electron is on the energy ladder, the farther it is from the nucleus. quantum of energy is the amount of energy required to move an electron from one energy level to another energy level. Nucleus
To move from one energy level to another an electron must gain or lose just the right amount of energy. The higher an electron is on the energy ladder, the farther from the nucleus.
The Bohr Model The Bohr model establishes the concept of definite electron energy levels within atoms. But Bohr's model was rather simplistic and as scientists made more discoveries about more complex atoms, Bohr's model was modified and eventually was replaced by more sophisticated models.
Models Of the Atom 1926- Erwin Schrodinger develops mathematical equations to describe the motion of electrons in atoms. His work leads to the electron cloud model.
The Quantum Mechanical Model The modern description of the electrons in atoms, the Quantum Mechanical Model, comes from the mathematical solutions to the Schrodinger equation. Like the Bohr model, the quantum mechanical model of the atom restricts the energy of electrons to certain values. Unlike the Bohr model, however, the QMM does not involve an exact path the electron takes around the nucleus.
Key Concept!!! The Quantum Mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus.
Here is a quantum mechanical picture of an Hydrogen atom. The nucleus is not shown, but is located at the center of the picture. Here are some things to notice: Like the heads you can see where the electron is most likely to be: near the nucleus (the center of the picture). You can't tell exactly where the electron is, just where it is most likely to be. The individual dots are not electrons. They are meant to be used in the context of how dense, or heavy an area of dots appears. The more crowded (or heavier packed) the dots are in a particular region, the better chance you have to finding your electron there.
Bohrs Atom - problems Only explain hydrogen spectrum. Could not explain molecules (bonding of atoms) - formation or properties. Why fixed orbits and no energy radiation in orbits. At variance with Heisenberg s uncertainty principle. Heisenberg: Not possible to know both the position and velocity of an electron at the same time with the same amount of accuracy.
The Successes and Limitations of the Bohr Atomic Model Bohr's realization that the atom's energy is quantized-that electrons are restricted to specific energy levels (orbits)-was an astounding achievement. this model successfully predicted the coloured lines in the visible-light portion of hydrogen's emission spectrum http://www.upscale.utoronto.ca/GeneralInterest/Harrison/BohrModel/Flash/Bohr Model.html
Bohrs model of atom explains emission spectrum of hydrogen other atoms however, posed problems http://www.upscale.utoronto.ca/GeneralInterest/Harrison/BohrModel/Flash/BohrModel. html
The Quantum Mechanical Model By the mid-1920s, it had become apparent that the Bohr model could not explain and make predictions about multi-electron atoms Three physicists: Schrodinger, de Broglie, and Heisenberg, developed to explain properties of matter called wave mechanics or commonly, quantum mechanics.
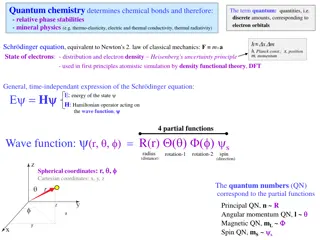
Schrodingers standing wave de Broglie originated the idea that the electron, previously consisted just as a particle , has wave properties Schrodinger approached the problem of atomic structure by focusing on the wave properties of the electron. In 1926, Schrodinger published an equation known as the Schrodinger wave equation.
Solution to Schrodingers equation gives many wave functions that describe various types of orbitals. Each of these types of orbitals has a set of four numbers called quantum numbers, which describe various properties of the orbital. they are denoted as n,l ml and ms when you substitute specific combinations of integers for each of these variables into the wave function, you have different solutions to the wave equation.
Quantum Numbers quantum numbers describe electrons in atoms three quantum numbers are used to describe the distribution of electrons in the atom the first quantum number, n., describes an orbitals energy level relative to size. the second quantum, l, and the and third quantum number, ml, respectively describe an orbital s shape and orientation in space. the fourth quantum number, ms, describes the behaviour of a specific electron in an orbital.
The Principal Quantum Number, n describes the size and energy of an atomic orbital has whole-number values (1,2,3, ) a higher value for n indicates a higher energy level. a greater n value also means the size of the shell is larger with a higher probability of finding an electron farther from the nucleus. the greatest number of electrons possible in any energy level is 2n2
The Secondary Quantum Number, l describes the shape of an atomic orbital. refers to energy sub levels, or subshells, within each principal energy level. the values of l are dependent on the value of the principal quantum number, n. values of l are positive integers that range in value from 0 to n-1 If n=2, l can be either 0 or 1 If n=3, l can be 0,1, or 2 Note: the number of possible values for l in a given energy level is the same as the value of n
In other words, if n=2 then there are only two possible sublevels, or subshells (2 types of orbital shapes) at this energy level. Each value of l is identified by a specific letter s, p, d, or f that is used to help distinguish it from the principal quantum number.
The l=0 orbital has a letter s The l=1 orbital has a letter p The l=2 orbital has a letter d The l=3 orbital has a letter f To identify an energy sublevel, combine the value of n with the letter of the orbital shape for example, the sublevel with n=3 and l=0 is called the 3s sublevel. The sublevel with n=2 and l=1 is the 2p sublevel
Magnetic Quantum Number, ml describes the orientation of the orbital in space around the nucleus. the number of different values that ml can have equals the number of orbitals that are possible. value of mldepends on the value of l. For any given value of l, there are (2l+1) values for ml ranging from l to + l
The Spin Quantum Number, ms related to the spin of an electron it specifies the direction in which the axis of an electron is oriented and has only two possible values, +1 2?? 1 2
in 1925, Wolfgang Pauli proposed that only two electrons of opposite spin could occupy an orbital. became known as the Pauli exclusion principle thus an orbital can have a maximum of two electrons only, each of which must have the opposite spin of the other. In a given atom, no two electrons can have the same set of four quantum numbers (n, l, ml, ms)
RULES AND PRINCIPLES ARISING FROM QUANTUM MECHANICS These four rules govern how electrons are arranged in atoms. HEISENBERG S UNCERTAINTY PRINCIPLE You cannot determine both the position and momentum of an electron at the same time. PAULI S EXCLUSION PRINCIPLE No two electrons can have the same four quantum numbers. THE AFBAU (BUILDING UP) PRINCIPLE Electrons enter the lowest available energy level. HUND S RULE OF MAXIMUM MULTIPLICITY When in orbitals of equal energy, electrons will try to remain unpaired.
ELECTRONS OCCUPY ORBITALS An orbital is a region in space where there is a 95% probability of finding the electron. (Heisenberg s Uncertainty Principle) Orbitals can hold one electron or two electrons as long as they have opposite spin. (Pauli Exclusion Principle) Orbitals have different shapes depending on which sub-shell they are in. DO NOT USE THE WORD ORBIT WHEN YOU MEAN AN ORBITAL
SHAPES OF ORBITALS One s orbital makes the s sub-shell spherical
SHAPES OF ORBITALS Three p orbitals make the p sub-shell dumb-bell shaped
SHAPES OF ORBITALS Five d orbitals make the d sub-shell four are double dumb-bell shaped the other a dumb-bell with a collar
Diagonal Rule for Build-up Rule 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 6f The periodic table can also be used to determine the electron configuration of an element.
Aufbau Diagram for Fluorine