Understanding Azeotropic Distillation Sequences in Chemical Engineering
Azeotropic distillation columns play a crucial role in separating complex mixtures containing azeotropes like alcohols, ketones, ethers, acids, and water. By studying phase diagrams, residue and distillation curves, and process flow diagrams, engineers can grasp the nuances of product composition control and system design in azeotropic distillation.
- Azeotropic Distillation
- Chemical Engineering
- Phase Diagrams
- Product Composition
- Process Flow Diagrams
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
SEQUENCING OF AZEOTROPIC DISTILLATION COLUMNS Ref: Seider, Seader and Lewin (2004), Product and process design principles, 2nd edition, Wiley, Section 7.5 1
Introduction Separation sequences are complicated by the presence of azeotropes, often involving mixtures of oxygenated organic compounds: Alcohols Ketones Ethers Acids Water In these cases, distillation boundaries limit the product compositions of a column to lie within a bounded region Prevent the removal of certain species in high concentrations 2
Instructional Objectives When you have finished studying this unit, you should: Be able to sketch the residue and distillation curves on a tertiary phase diagram Be able to define the range of possible product compositions using distillation, given the feed composition and the tertiary phase diagram Be able to define the PFD for a heterogeneous azeotropic distillation system Be able to define the PFD for a pressure swing distillation system 3
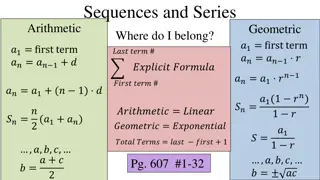
Homogeneous Azeotropes At equilibrium: V f j j y P yP yP L _ _ f x f xP xP = = j j V L j j j = = s 1 1 1 s 2 2 2 P xP P xP P xP ( 1 x P ) = + = + s s s s 1 1 2 2 1 1 1 2 ( P x ) = + s s s 2 1 2 1 At fixed temperature 4
Homogeneous Azeotropes (Contd) Example Phase diagrams for benzene-toluene mixture at 90 oC 5
Homogeneous Azeotropes (Contd) yP yP x P x = = S For non-ideal mixtures, the activity coefficients are different from unity: 1 1 1 1 P S 2 2 2 2 P x P ( 1 x ) P = + s s 1 1 1 1 2 2 1 If the mixture has a minimum-boiling azeotrope i Example Phase diagrams for Isopropyl ether-Isopropyl alcohol 6
Homogeneous Azeotropes (Contd) yP yP x P x = = S For non-ideal mixtures, the activity coefficients are different from unity: 1 1 1 1 P S 2 2 2 2 P x P ( 1 x ) P = + s s 1 1 1 1 2 2 1 If the mixture has a maximum-boiling azeotrope i Example Phase diagrams for Acetone-Chloroform 7
Heterogeneous Azeotropes For a minimum-boiling azeotrope with large deviation from Raoult s law ( ), phase splitting may occur and a minimum-boiling heterogeneous azeotrope forms, having a vapor phase in equilibrium with two liquid phases. i usually , 1 7 Homogeneous Azeotrope Heterogeneous Azeotrope 8
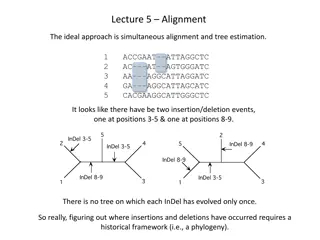
Residue Curves Distillation still Mass balance on species j: Lx L y ( ) = + ( L L x )( x ), j 1 , , C 1 + = j j j j As L 0: Lx ydL Lx Ldx dx dL xdL dLdx j , 1 , , C 1 = + + = j j j j j j Rearranging: x y x 1 ( K T P x y { , , , }) = = j / L j j j j dx dt x y = j j j 9
Residue Curves (Contd) dx dt x y = j j j Residue curves for zeotropic system Residue curves for Azeotropic system 10
Liquid Compositions at Total Reflux Species balance on top n-1 trays: L x Dx Approximation for liquid phase: Vy + = n 1 n 1 n n D dx dh x x n n n 1 Substituting: dx dh At total reflux, D = 0 and Vn = Ln-1 dx x dh V L D L x y x + n n n n D n 1 n 1 Stripping section of distillation column y n n n The residue curves approximates the distillation curves at total reflux 11
Distillation Curves An exact representation of the distillation curve (operating line) at total reflux can be calculated based on: 1- Between two adjacent stages: = , 1 , 0 = x y n n + 1 the n 2- On each stage: and lie at the ends of equilibriu m tie lines x y n n 12
Comparison of Residue and Distillation Curves When the residue curve is linear (as for binary mixtures), the distillation and residue curves are coincide. Their differences are pronounced in the regions with extensive curvature. 13
Sketching Residue Curves (Exercise) dx dt x y = j j j 15
Product Composition Regions for Zeotropic Systems 16
Product Composition Regions for Azeotropic Systems 17
Heterogeneous Azeotropic Distillation Example: Dehydration of Ethanol Try toluene as an entrainer 18
D1 M2 M1 S1 S2 D2 19
Pressure-swing Distillation (Contd) Example: Dehydration of Tetrahydrofuran (THF) T-x-y diagrams for THF and water 21
Azeotropic Distillation - Summary On completing this unit, you should: Be able to sketch the residue curves on a tertiary phase diagram Be able to define the range of possible product compositions using distillation, given the feed composition and the tertiary phase diagram Be able to define the PFD for a heterogeneous azeotropic distillation system Be able to define the PFD for a pressure swing distillation system 22