Understanding Coal Desulfurization Process
Coal is a crucial energy source, especially in developing countries. However, the burning of coal releases sulfur oxides, a significant pollutant. Desulfurization methods are essential to reduce harmful emissions and protect the environment and human health. This article discusses the importance of desulfurization in coal, the types of sulfur in coal, and the challenges associated with sulfur emissions from burning coal.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
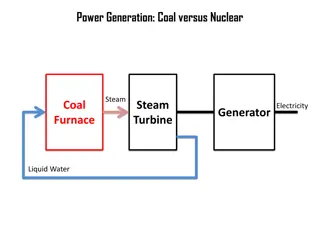
1.Introduction Coal is one of the main sources of world energy.Economic growth and industrialization in developing countries are leading to a rapid increase in the demand for energy; in other words, an increased demand in the use of coal, which is inexpensive and readily available , for electric power and process heat.
2.Lignite Lignite is a type of coal which is typically used as fuel for thermal power plants since it has low heating value, and high ash and humidity content. Nevertheless it is an energy raw material that is used frequently due to its abundance in the earth's crust.
Hard coal is classified as a high-calorie coal. Of our domestic resource potential, 12,4 billion tons is lignite, and 1,33 billion tons is hard coal. The most important hard coal reserves of our country are in Zonguldak and its vicinity. The total hard coal reserve in Zonguldak Basin is 1,322 billion tons, while visible reserve here is at the level of 519 million tons. (Hard Coal Sector Report 2009)
TABLE 1: Distribution of newfound lignite reserves by regions, as of May 2008 Lignite Reserve Regions in Turkey Reserve Amount Afsin-Elbistan* 1.915 million tons Elbistan* 420 million tons Konya-Karapinar 1.280 million tons Thrace 498 million tons Manisa-Soma-Eynez 170 million tons Eski ehir-Alpu 275 million tons
3.Sulfur in coal When coal is burned, generally 90% or more of the sulfur present in it is emitted into the atmosphere as sulfur oxides (mainly SO2), if no desulfurization methods are used before, during and after combustion.
SOx is most important pollutant as a real treat to both the ecosystem and human health. Sulfur types in coal : 1)Inorganic sulfur Sulfate-S, Pritic-S, Elemental-S, 2)Organic sulfur
4.How do you make coal cleaner? Actually there are several ways.Takesulfur, for example. Sulfur is a yellowish substance that exists in tiny amounts in coal. Still, it is important that most of this sulfur be removed before it goes up a power plant's smokestack.
One way is to clean the coal before it arrives at the power plant. One of the ways this is done is by simply crushing the coal into small chunks and washing it. Some of the sulfur that exists in tiny specks in coal It is called PYRITIC SULFUR.
Not all of coal's sulfur can be removed like this, however. Some of the sulfur in coal is actually chemically connected to coal's carbon molecules instead of existing as separate particles. This type of sulfur is called "ORGANIC SULFUR," and washing won't remove it.
Most modern power plants and all plants built after 1978 are required to have special devices installed that clean the sulfur from the coal's combustion gases before the gases go up the smokestack. The technical name for these devices is "flue gas desulfurization units," but most people just call them "scrubbers" because they "scrub" the sulfur out of the smoke released by coal-burning boilers.
5.How do scrubbers work? Most scrubbers rely on a very common substance found in nature called "limestone." We literally have mountains of limestone throughout this country. When crushed and processed, limestone can be made into a white powder. Limestone can be made to absorb sulfur gases under the right conditions much like a sponge absorbs water.
How the Wet Flue Gas Desulfirization Technology Works
6.Syngas Syngas is the abbreviation for Synthesis gas. This is a gas mixture that comprises of carbon monoxide, carbon dioxide and hydrogen. The syngas is produced due to the gasification of a carbon containing fuel to a gaseous product that has some heating value.
Some of the examples of syngas are as follows gasification of coal, waste to energy gasification, steam reforming of natural gas to generate hydrogen. Syngas has 50% the energy density of natural gas. It can be burnt and is used as a fuel source.
The other use is as an intermediate to produce other chemicals. The use of syngas as a fuel is accomplished by the gasification of coal or municipal waste. There are commercially available technologies to process syngas to generate industrial gases, fertilizers, chemicals, fuels and other products.
References http://www.enerji.gov.tr/index.php?dil=en&sf=webpa ges&b=komur_EN&bn=511&hn=&nm=40717&id=40729 http://www.netl.doe.gov/technologies/coalpower/cctc /coal101/coal101-2.html http://www.eolss.net/sample-chapters/c08/e3-04-02- 04.pdf http://www.iit.edu/wiser/research/projects/images/cl ean_coal_flow.jpg http://biofuel.org.uk/what-is-syngas.html
Thanks for listening Havvanur BALCI rem Ay a KAVAKLIO ULLARI