Understanding Naming Ionic Compounds and Formulas
Ions and salts play a crucial role in forming solid compounds known as salts. By following specific rules, you can name ionic compounds based on the cation and anion present. Determining formulas involves identifying charges and balancing them. Explore the process through examples like KCl, MgO, AlCl3, CaF2, and more.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
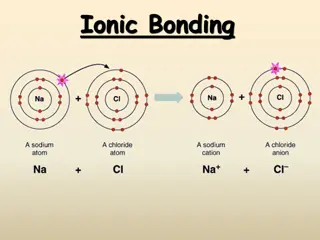
Ions and salts Ions can be single atoms, as the sodium and chlorine in common table salt (sodium chloride), or more complex (polyatomic) groups such as the carbonate in calcium carbonate An ionic bond, one bonder must have a positive charge and the other a negative one Most cations and anions can combine to form solid compounds that are usually known as salts and their net charge must be neutral
ionic compound is named first by its cation and then by its anion Naming Ionic Compounds For example, K+1 is called the potassium ion, just as K is called the potassium atom cation has the same name as its element
anion is named by taking the elemental name, removing the ending, and adding -ide For example, F-1 is called fluoride, for the elemental name, fluorine. -ine was removed and replaced with -ide Naming Ionic Compounds To name a compound, the cation name and the anion named are added together For example, NaF is also known as sodium fluoride
Try naming the Compounds! KCl MgO AlCl3 CaF2
Determining the formula for Magnesium Fluoride? Identify the charges = Mg2+ F-1 Cross the Charges, Mg2+F-1 If the subscript is a 1 it does not need to be written. If there is a common subscript such as 2 as in Mg2O2, reduce it to Mg1O1which is also MgO. = Mg1F2
Try it! Sodium Chloride Aluminum Chloride Magnesium Nitride
Ionic compounds With Metals Iron (III) indicates a +3 charge Lead (V) indicates a +5 charge
Name to Formula Nickel (II) Chloride Cr (IV) Oxide
Formula to Name Fe2O3 Y3N3