ASQ Pharmaceutical GMP Professional (CPGP) Exam | Boost Your Score
Click Here---> //bit.ly/4bsglWA
- ASQ
- CPGP pdf
- CPGP questions
- CPGP exam guide
- CPGP practice test
- CPGP books
- CPGP tutorial
- CPGP syllabus
- Auditing
- ASQ Pharmaceutical GMP Professional Exam Questions
- ASQ Pharmaceutical GMP Professional Question Bank
- ASQ Pharmaceutical GMP Professional Questions
- ASQ Pharmaceutical GMP Professional Test Questions
- ASQ Pharmaceutical GMP Professional Study Guide
- ASQ CPGP Quiz
- ASQ CPGP Exam
- CPGP
- CPGP Certification
- CPGP Body of Knowledge (BOK)
Download Presentation
Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
Presentation Transcript
How to Prepare for ASQ CPGP Exam?
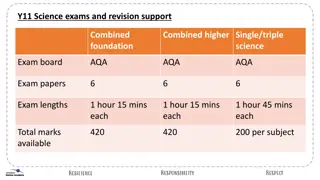
ASQ CPGP Exam Summary: Vendor ASQ Exam Code CPGP Full Exam Name ASQ Certified Pharmaceutical GMP Professional Number of Questions 165 Sample Questions ASQ Pharmaceutical GMP Professional Exam Sample Questions and Answers ASQ Certified Pharmaceutical GMP Professional (CPGP) Practice Test 550/750 Practice Exam Passing Score Time Limit 258 Minutes
CPGP Certification Syllabus Content: Syllabus Topics: Regulatory Agency Governance Materials and Supply Chain Management Quality Systems Sterile and Nonsterile Manufacturing Systems Laboratory Systems Filling, Packaging, and Labeling Infrastructure: Facilities, Utilities, and Equipment Product Development and Technology Transfer
ASQ CPGP Training: Recommended Training: CPGP Handbook
How much Fees of ASQ CPGP Exam? ASQ CPGP Fee Structure: ASQ MEMBERS - $433 NON-MEMBERS - $533 RETAKES - $333
CPGP Study Guide: Know about ASQ Pharmaceutical GMP Professional book details. Go through ASQ CPGP exam syllabus. Go through ASQ Pharmaceutical GMP Professional sample questions. This will give you a clear idea about the real exam. Enroll for CPGP practice test on ProcessExam.com. Identify your weak areas from CPGP sample exam and do more practice with system.
ASQ Pharmaceutical GMP Professional Sample Questions
Que.: 1. During laboratory investigations, _______ methods are reviewed to ensure they are verified as suitable for use in the testing lab. Options: a) compendial b) outdated c) unverified d) improvised
Answer: a) compendial
Que.: 2. Environmental conditions such as temperature and humidity are controlled during the storage of materials to: Options: a) Make the materials easier to transport b) Prevent unauthorized access to the materials c) Maintain the materials characteristics and ensure accurate testing results d) Reduce the volume of materials stored
Answer: c) Maintain the materials characteristics and ensure accurate testing results
Que.: 3. Health Canada is the federal department responsible for helping Canadians maintain and improve their health. In which country does it operate? Options: a) United States b) Australia c) Canada d) United Kingdom
Answer: c) Canada
Que.: 4. PIC/S guidelines emphasize the importance of data integrity. Which of the following is a key aspect of these guidelines? Options: a) Data should be easily editable by anyone. b) Data should be reproducible under similar conditions. c) Records must be stored in a public cloud for accessibility. d) Documentation practices must prevent data tampering.
Answer: d) Documentation practices must prevent data tampering.
Que.: 5. ICH Q10 integrates elements of _____ and continual improvement into the quality management system. Options: a) profit maximization b) product lifecycle c) competitive analysis d) investor relations
Answer: b) product lifecycle
Unique Features Continued. ProcessExam.com has provided good quality CPGP sample questions. One can go through the Pharmaceutical GMP Professional sample questions before buying the CPGP online practice test. One can take unlimited attempts to practice from the CPGP practice test. It is available for two months. A candidate is able to measure his speed from the online practice test. Best CPGP book links are also provided on the website syllabus page.
Unique Features Continued. If a candidate wants to know about Pharmaceutical GMP Professional training detail, our website provides information about that too. A candidate is able to know about his performance depending on the result section of Pharmaceutical GMP Professional online test. Marks obtained could be a motivator factor to prepare more or less depending on the result. Last but not the least, we have a money back policy in our website,that makes us really unique. Testimonials written on the website, could be helpful to choose our website, as these are shared by our valuable users, who availed our online practice test.
To Know More about ASQ CPGP Certification VISIT www.processexam.com