Advances in Ring-Closing Metathesis and Cross-Metathesis Catalysts
Recent developments in metathesis catalysts, focusing on Molybdenum and Ruthenium-based catalysts. Comparison of Schrock and Grubbs catalysts, ligands, and new modified catalysts. Details on activity, stability, and group tolerance. The potential of new catalysts like Piers II, Grubbs III, nitro-Grela, and Stewart-Grubbs in perspective with ruthenium catalysts. A comprehensive overview of catalyst evolution in the field.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
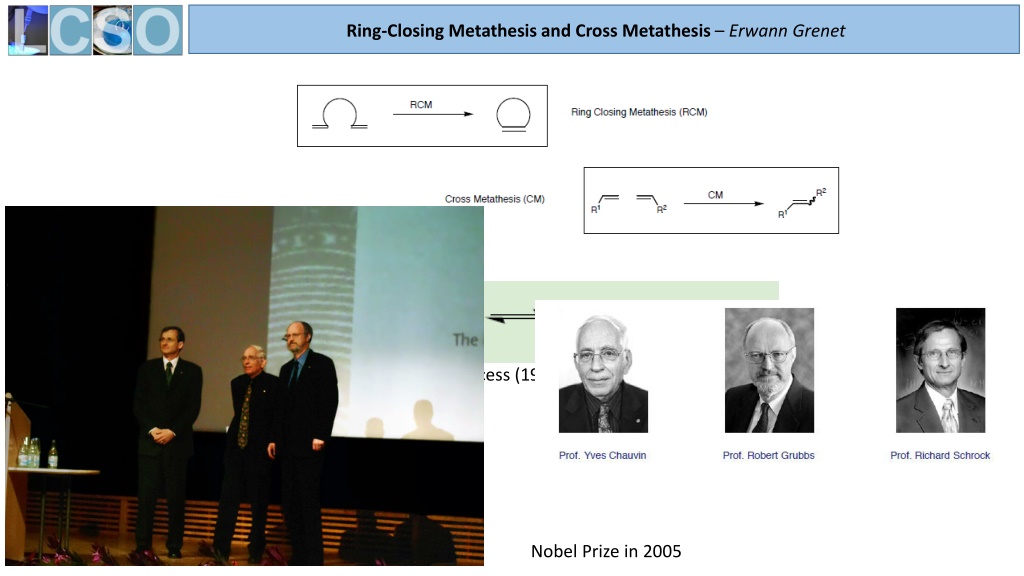
Ring-Closing Metathesis and Cross Metathesis Erwann Grenet Phillips Triolefin Process (1964) with Mo(CO)6 AlO2, WO3 SiO2 Nobel Prize in 2005
Ring-Closing Metathesis and Cross Metathesis Erwann Grenet Overview of the available catalysts 167 CHF/g 3505 582 CHF/g 3279 Price on Sigma-Aldrich Number of ref on SciFinder 3500 CHF/g 315 392 CHF/g 1392 612 CHF/g 343 For Mo-Schrock: Schrock, R. R.; Murdzek, J. S.; Bazan, G. C.; Robbins, J.; DiMare, M.; O'Regan, M. J. Am. Chem. Soc. 1990, 112, 3875 3886. For G-I : Schwab, P.; France, M.B.; Ziller, J.W.; Grubbs, R.H. Angew. Chem. Int. Ed. 1995, 34, 2039-2041. For G-II : Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H., Tetrahedron Lett. 1999, 40, 2247-2250. For GH-I: Kingsbury, J. S.; Harrity, J. P. A.; Bonitatebus, P. J.; Hoveyda, A. H. J. Am. Chem. Soc. 1999, 121, 791 799. For GH-II: Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2000, 122, 8168 8179.
Ring-Closing Metathesis and Cross Metathesis Erwann Grenet Molybdenum-based catalysts Ruthenium-based catalysts Schrock vs Grubbs ACTIVITY STABILITY Efficient for very hindered olefin Alkyne metathesis Broad tolerance Non tolerant to oxo functionnal groups (carbonyl, hydroxyl, etc) Non tolerant to phosphine, sulfure or electron-rich amine groups = < They are the two wings of the same angel. The angel can't fly if you clip either one. Amir Hoveyda
Ring-Closing Metathesis and Cross Metathesis Erwann Grenet Phosphine ligands NHC ligands 1stgener. vs 2ndgener. Similar group tolerance and thermal stability Better catalytic activity Phosphine ligands Phosphine-free ligands - Faster kinetic Grubbs vs Hoveyda Trouble with sulfure group -
Ring-Closing Metathesis and Cross Metathesis Erwann Grenet The new modified catalysts 605 CHF/g 24 Piers II (P-II) Grubbs III (G-III) 690 CHF/g 96 2924 CHF/g 456 nitro-Grela 555 CHF/g 456 Stewart-Grubbs New battle in perspective with ruthenium catalysts? Mo-catalyst in paraffin pellets For G-III : Sanford, M. S.; Love, J. A.; Grubbs, R. H. Organometallics 2001, 20, 5314 5318. For Piers: Romero, P. E.; Piers, W. E.; McDonald, R. Angew. Chem. Int. Ed. 2004, 43, 6161-6165. For Stewart-Grubbs: Stewart, I. C.; Douglas, C. J.; Grubbs, R. H. Org. Lett. 2008, 10, 441-444. For nitro-Grela: Angew. Chem. Int. Ed. 2002, 41, 4038-4040. For Schrock paraffin pellets: Nguyen, T. T., Koh, M. J.; Shen, X.; Romiti, F.; Schrock, R. R.; Hoveyda, A. H. Science 2016, 352, 569-574.
167 CHF/g 3505 392 CHF/g 1392 582 CHF/g 3279 612 CHF/g 343 3500 CHF/g 315 555 CHF/g 456 2924 CHF/g 160 605 CHF/g 24 690 CHF/g 96 nitro-Grela Piers II (P-II) Stewart-Grubbs Price on Sigma-Aldrich Number of ref on SciFinder For Mo-Schrock: Schrock, R. R.; Murdzek, J. S.; Bazan, G. C.; Robbins, J.; DiMare, M.; O'Regan, M. J. Am. Chem. Soc. 1990, 112, 3875 3886. For G-I : Schwab, P.; France, M.B.; Ziller, J.W.; Grubbs, R.H. Angew. Chem. Int. Ed. 1995, 34, 2039-2041. For G-II : Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H., Tetrahedron Lett. 1999, 40, 2247-2250. For HG-I: Kingsbury, J. S.; Harrity, J. P. A.; Bonitatebus, P. J.; Hoveyda, A. H. J. Am. Chem. Soc. 1999, 121, 791 799. For HG-II: Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2000, 122, 8168 8179. For G-III : Sanford, M. S.; Love, J. A.; Grubbs, R. H. Organometallics 2001, 20, 5314 5318. For nitro-Grela: Angew. Chem. Int. Ed. 2002, 41, 4038-4040. For Piers: Romero, P. E.; Piers, W. E.; McDonald, R. Angew. Chem. Int. Ed. 2004, 43, 6161-6165. For Stewart-Grubbs: Stewart, I. C.; Douglas, C. J.; Grubbs, R. H. Org. Lett. 2008, 10, 441-444. Schrock HG-I HG-II Grela S-G 1990 1995 G-I 1999 G-II 2000 2001 G-III 2002 2004 P-II 2008