Elephant Toothpaste Experiment - Investigating Catalysts in Chemical Reactions
This extended experimental investigation explores the breakdown of hydrogen peroxide using different catalysts to create 'elephant toothpaste.' The experiment aims to determine which catalyst is most efficient at catalyzing the reaction, involving yeast, magnesium dioxide, and potatoes. Background research, hypothesis, equipment list, and methodology are presented, culminating in a hands-on exploration of chemical reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
CBA 1 CBA 1- - Extended Extended Experimental Experimental Investigation Investigation Elephant toothpaste experiment- chemical reactions By Rafia Nauman
Table of Table of Contents! Contents! Part A Part A- - Part C Part C- - Questioning and Predicting Processing and Analysing Part B Part B- - Part D Part D- - Planning and Conducting Reflecting and Reporting
01 Part A Part A- - Questioning and Predicting
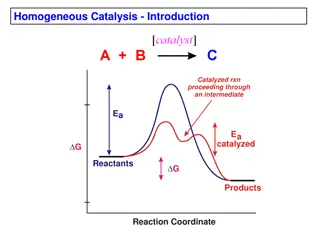
Background research Background research When yeast (a catalyst) is mixed into hydrogen peroxide it causes a rapid reaction, the hydrogen peroxide breaks down into large amounts of water and oxygen, this causes the mixture to quickly expand and burst out. When washing up liquid is added to the solution it turns it into a foam by trapping the oxygen bubbles.
Background research Background research Yeast is just one of the many possible catalysts that can be used to catalyse the breakdown of hydrogen peroxide eg. Potatoes, magnesium dioxide. In our experiment we are trying to see what catalyst breaks down H O the best so we ll be using multiple catalysts to see which one is the most efficient at creating foam.
Question Topic- Chemical Reactions Which substance catalyses the breakdown of hydrogen peroxide the best*
If the catalyst is from a laboratory then the breakdown of hydrogen peroxide would be the best because catalysts are specifically for reactions Hypothesis Hypothesis
02 Part B Part B- - Planning and Conducting
Equipment: Equipment: Graduated cylinder Graduated cylinder Gloves Gloves Dish soap Dish soap Safety goggles Safety goggles Food colouring Food colouring Hydrogen peroxide Hydrogen peroxide Water Water Spatula Spatula Catalysts: Catalysts: Yeast Yeast Magnesium dioxide Magnesium dioxide Potatoes Potatoes Plastic tub Plastic tub Stirring rod Stirring rod Beaker(for water) Beaker(for water) Beaker(for H O ) Beaker(for H O ) 2 2 2 2
Safety Safety Clothes Clothes Hydrogen peroxide can bleach clothing so I have to be careful when dealing with the acid Gloves Gloves To protect my hands as I ll be working with hydrogen peroxide which is a corrosive acid Plastic tub Plastic tub I ll do the reaction in a plastic tub to protect the table and myself Safety goggles Safety goggles To protect my eyes as hydrogen peroxide can irritate them
Variables and Variables and Controls Controls Constants Volume of hydrogen peroxide Volume of the catalyst Volume of dish soap Time (1 minute) Temperature Volume of dish soap Independent Variable The catalyst will be the independent variable Dependant Variable How much foam is produced will be the dependant variable
Good experimental Good experimental design design I will conduct the experiment 3 times with each catalyst so then I can calculate an average to get a more accurate result I will be careful when pouring and measuring the catalysts, hydrogen peroxide and dish soap so that the experiment stays mainly constant other than my own added variable, the catalyst I will keep everything room temperature to avoid introducing a new variable Gloves for safety Carefully measuring everything
Procedure Procedure Step 1- gather equipment and measure accurate volumes of dish soap(2 teaspoons),water(50ml), catalyst(10g) and hydrogen peroxide(50ml). Put on gloves and safety goggles. Step 5- pour the catalyst solution into the graduated cylinder Step 6- for my experiment since I m trying to see which substance catalyses the breakdown of hydrogen peroxide the best I will immediately start a stopwatch and stop it at exactly 1 minute and record the volume of foam produced Step 2- place the graduated cylinder in the plastic tub and pour the hydrogen peroxide into the graduated cylinder. Step 3- put the catalyst into the beaker and mix with some water(grind the catalyst with a mortar and pestle if needed) Step 7- repeat this process with each catalyst three times and record the foam produced 1 minute after the initial reaction. Step 4- squirt a drop of dish soap into the graduated cylinder and mix with stirring rod.
Diagram Diagram Here is a diagram of what the experiment looks like, I pour the beaker with the catalyst mixed with the water into the graduated cylinder and the reaction happens, I time it and at one minute I record the volume of foam.
03 Part C Part C- - Processing and Analysing
Results Results As you can see there is a drastic change in foam produced from the first time I did the experiment to the second and last. This could be due to the fact that I did the first experiment with the potato on a Monday and then only 3 days later on Thursday did I do the other three, the drastic change in foam could suggest that the ripeness of the potato plays a key role in the reaction. This will definitely affect the accuracy of the overall average and experiment. For my experiment I decided to test the breakdown of hydrogen peroxide with 3 different catalysts, potatoes, magnesium dioxide and yeast. Here are the results: Potato Potato The volume of foam produced from using a potato as a catalyst was 320cm2,130cm2and 190cm2.
Later my teacher gave me a different pack of magnesium dioxide, after performing the experiment and recording the data we came to the conclusion that the pack was kept in storage for longer and must have oxidized, introducing an unexpected variable. Magnesium dioxide Magnesium dioxide The volume of foam produced using magnesium dioxide was 920cm2, 940cm2,and 540cm2. As you can see there is another drastic change in the volume of foam, again this is due to another unwanted and unexpected variable. For the first and second time I performed the experiment I used magnesium dioxide I borrowed from my science teacher however there was only enough for two experiments.
Yeast Yeast The volume of foam produced by using the yeast was 120cm2, 1080cm2,and 960cm2. It s quite obvious that something must ve interfered and introduced a new variable during the reaction, there was a huge change in volume when I did the experiment the second and third time. This is most likely due to the fact that I used different brands of yeast, thinking that it wouldn t affect the reaction however I was sorely mistaken.
Results Results Catalyst 1st experiment 2nd experiment 3rd experiment Calculatio ns Mean Average . Potato 320 130 190 320+130+19 0= 640 3= 213.3= 213cm2 Magnesium dioxide 920 940 540 920+940+54 0= 2400 3= 800cm2 Yeast 120 1080 960 120+1080+9 60= 2160 3= 7202
Results Results . Here are the three results for each catalyst in bar chart form, I thought this would be the best way to display this data. Here are the mean average results in bar chart form.
Patterns/ Patterns/ Relationships Relationships By analysing the data I can determine that substances that I derived from the school laboratory catalysed the breakdown of hydrogen peroxide better than the potato which is a natural substance.
Conclusion Conclusion By looking at the graphs I can see that the potato reacted quite poorly compared to the yeast and magnesium dioxide. This means that a natural catalyst does not catalyse the breakdown of hydrogen peroxide the best but rather yeast and magnesium dioxide. Overall the magnesium catalysed the breakdown of hydrogen peroxide the best by producing the most foam. However, I am aware that due to various problems with performing the experiments this information might not be the most accurate.
Has my hypothesis been supported? Yes, my hypothesis has been supported, it was that if the catalyst is from a laboratory then the breakdown of hydrogen peroxide would be the best and it was. Both the laboratory substances used, yeast and magnesium dioxide had moderately better reactions compared to the potato
04 Part D Part D- - Reflecting and Reporting
Design of the Design of the experiment experiment However for this experiment be as accurate as possible i had to be extremely careful not to introduce another variable which unfortunately did occur. Even if the execution had some difficulty the overall design I believe was quite good. The experiment was to do an elephant toothpaste reaction with different catalysts and then comparing them to see which does the best at producing the most foam, 1 minute after the initial reaction. I think that the design of the experiment was quite good, it was easy to understand and perform after some practice.
Sources of Sources of error error but the conclusion that laboratory sourced substances produce more foam might be incorrect as I didn t investigate that many possible catalysts. There are definitely going to be errors in my investigation since I had limited time and supplies. The accuracy in measuring the dish soap, hydrogen peroxide and catalyst was to my best ability,
Relationship to real Relationship to real life life 24-year-old biochemist Camille Schrier won Miss Virginia after an elephant toothpaste demonstration This experiment has little to no relationship at all with real life, elephant toothpaste is a reaction commonly used in classrooms as a demonstration of an exothermic reaction. More recently, it's been used at the 2019 Miss Virginia pageant and for gender reveals. I suppose if you were looking for a flashy gender reveal this investigation might ve been somewhat helpful. Limitations of data Limitations of data A major limitation I had with the data was that there wasn t much to compare with if I had perhaps investigated more catalysts then I think my final deduction would ve been much more accurate.
Possible Possible improvements improvements Possible extensions Possible extensions If i was to do this experiment again I would be much more careful with what catalysts I use and to make sure they are fresh and not to use different brands of yeast as that does affect the experiment. I also only dealt with a small sample group of just three catalysts if I had more time it would ve been best try more catalysts to make the conclusion as accurate as possible. As I previously mentioned I only dealt with a small sample group of catalysts and a good extension could be to add more into the investigation, it would be interesting to see how other catalysts such as radish and liver react to hydrogen peroxide and dish soap.
References References https://en.wikipedia.org/wiki/Elephant%27s_too thpaste https://mymodernmet.com/miss-virginia-science- talent/ https://www.scientificamerican.com/article/mak e-elephant-toothpaste/ https://kids.nationalgeographic.com/books/arti cle/elephant-toothpaste https://sciencenotes.org/elephant-toothpaste- two-ways-to-make-it/ Oide Kirwan
The End The End CBA by Rafia Nauman