Understanding Thermochemistry: Heat, Temperature, and Enthalpy
Thermochemistry is the study of heat energy associated with chemical and physical changes. It involves understanding concepts like heat transfer, temperature, enthalpy, and specific heat capacity. By exploring these principles, we can analyze heat changes in systems and calculate energy requirements for various processes, such as combustion reactions and temperature changes of substances like water or copper.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Thermochemistry Thermochemistry
What does the prefix thermo thermo mean? Thermochemistry is the study of the heat associated with a chemical or physical change. Heat Temperature Heat (symbolized by Q or H) is transferable radiant energy (enthalpy) Units joules (J), kilojoules (kJ), calories (cal) and kilocalories (kcal) = Calories (Cal) Food calories 4.184 J = 1 cal
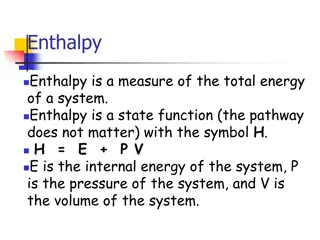
When we study Heat of a System it is important to know if heat is increasing or decreasing Heat is a product Exothermic = Enthalpy OUT (Heat) Heat is a reactant Endothermic = Enthalpy IN (Heat)
When we study Heat of a System it is important to know if heat is increasing or decreasing
Heat Temperature Heat Temperature Temperature (T) is a measure of the differences in enthalpy between two objects Units Degree Celsius or Kelvin How much Enthalpy (heat) in joules does the combustion of methane release if it releases 45 calories of heat energy?
The relationship between Heat from temperature is muddled: Depends on what substance how much substance and how much energy is available . Specific Heat Capacity (Cp): The amount of heat required to raise the temperature of 1 gram of substance by 1 degree Celsius Units: J g C g C or cal ** Specific Heat of water is 4.184 J/g C or 1 cal/g C **
Q is heat change as a result of a temperature change Calorimeter = tool used to measure heat changes Q = mass x change in Celsius temperature x specific heat capacity of the substance Q = m (Tf Ti) Cp Q = mC T Example: How much heat is required to raise the temperature of 2.5g of water by 12.5 C?
Complete the following specific heat practice problems in your notebook. 1. 5.0 g of copper was heated from 20 C to 80 C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g C) 2. How much heat is absorbed by a 20g granite boulder as energy from the sun causes its temperature to change from 10 C to 29 C? (Specific heat capacity of granite is 0.79 J/g C) 3. How much heat is released when 30 g of water at 96 C cools to 25 C?
1.5.0 g of copper was heated from 20C to 80C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g C) Q = mC T = 5.0g (0.092 cal/g C) (80 20) = 27.6 30 cal
2. How much heat is absorbed by a 20g granite boulder as energy from the sun causes its temperature to change from 10 C to 29 C? (Specific heat capacity of granite is 0.79 J/g C) Q = mC T = 20g (0.79 J/g C) (29-10) = 300.2 300 J
3. How much heat is released when 30 g of water at 96 C cools to 25 C? Q = mC T = 30g (4.184 J/g C) (25-96) = -8911 -9000 J