Thermochemistry Problem Practice and Solutions
Practice and solutions for thermochemistry problems involving energy calculations, temperature conversions, and phase changes. Includes calculations for melting ice, converting temperature to joules, and determining energy required for heating substances.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Answers to the 12 Thermochem Questions you practiced while walking around Grade yourself harshly, units and SF count dearly.
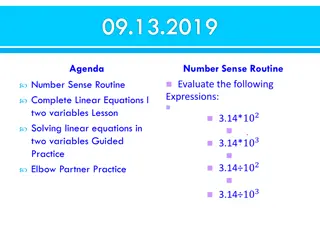
1. How much energy in joules, is required to melt 145 grams of solid ice at 273 K into liquid water? q = mHF = (145g)(334 J/g) = 48,400 J with 3 SF
2. Convert 1895 C into joules. X1000 cal 1 C 1895 C 1 = 1,895,000 cal X 4.18 J 1 cal 1,895,000 cal 1 = 7,921,000 J with 4 SF
3. How much energy in joules, is release when 432 grams of steam condenses into water? q = mHV = (432g)(2260 J/g) = 976,000 J with 3 SF
4. Copy down the 2 correct math expressions C of Cu > C of H2O HV = HF HV < HF C of Cu < C of H2O CICE < CWATER CICE > CWATER
5. How much energy in joules, is required to raise the temperature of 75.0 grams of water from 34.5 C to 45.8 C? q = mC T = (75.0g)(4.18J/g K)(11.3 K) = 3542.55 = 3540 J with 3 SF
6. Copy down the 2 correct math expressions HV > HF HV < HF HV = HF KE > PE CICE = CWATER CICE < CWATER
7. How much energy in joules, is required to raise the temperature of 75.0 grams of copper from 44.5 C to 55.8 C? (CCu = 0.391 J/g K) q = mC T = (75.0g)(0.391J/g K)(11.3 K) = 331.3725 = 331 J with 3 SF
8. Convert the number of joules in your answer to question #7 into kilojoules and into calories (small c ) 1 kJ 1000 J 331 J 1 = 0.331 kilojoules X 1 cal 4.18 J 331 J 1 = 79.2 cal X
9. At what temperature in CENTIGRADE would aluminum melt? K = C + 273 933 = C +273 -273 = -273 660 C
10. The HF for aluminum is 403 J/g, which is why you can t melt aluminum in your mouth. A soda can might mass at 48.2 grams. How much energy in joules is needed to melt that can into liquid? (assume T = 0) q = mHF = (48.2g)(403 J/g) = 19,424.6 = 19,400 J with 3 SF
11. When 454 g bismuth changes temperature from 273 K to 296 K, it takes 1284 Joules. What is the C of Bi? q = mC T 1284 J = (454g)(CBi)(23.0K) 1284 J 10,442 g K = CBi = 0.123 J/g K
The 13th Amendment to the Constitution of the United States "Neither slavery nor involuntary servitude, except as a punishment for crime whereof the party shall have been duly convicted, shall exist within the United States, or any place subject to their jurisdiction."
12. The CFe = 0.45 J/gK. When 2005 Joules is able to change the temperature of iron by 67.5 Kelvin, what is the mass of this iron? q = mC T 2005 J = (m)(0.45J/g K)(67.5K) 2005 J (30.375J/g) = 66.0 g with 3 SF the J s cancel out, the g of the denominator of the denominator jumps to the numerator The K s cancel out