Understanding the Wave Theory of Light and Electromagnetic Spectrum
Explore the fascinating world of electromagnetic waves, visible light, and the wave theory through concepts such as wavelength, frequency, amplitude, and the speed of light. Understand how these elements are interconnected, and discover the diverse range of the electromagnetic spectrum. Dive into the relationship between wavelength and frequency and delve into practical examples to solidify your understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Electronic Structure and the Periodic Table Unit 6 Honors Chemistry
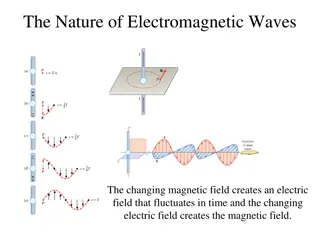
Wave Theory of Light James Clerk Maxwell Electromagnetic waves a form of energy that exhibits wavelike behavior as it travels through space Visible light a form of electromagnetic radiation that is perceivable to human beings and is seen in the colors of the rainbow ROY G. BIV
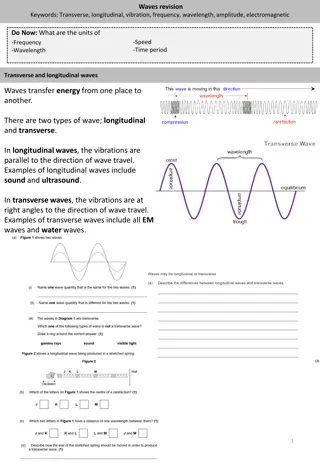
Wave Vocab: Crest the top of a wave Trough the bottom of a wave Wavelength ( crest to crest or trough to trough in a wave Units: m, nm (1 m = 109nm) lambda ) the distance from Frequency ( that pass a given point in a set amount of time (generally in 1 second) Units: Hertz (Hz), 1/s, or s-1 nu ) the number of wavelengths
Wave Vocab: Amplitude the distance from the origin to the crest or the trough of a wave Height (or intensity/brightness) of wave Speed of light (c) the rate at which all forms of electromagnetic radiation travel through a vacuum = 3.00 x108m/s
Comparing Waves As Wavelength increases, frequency _______________. As Wavelength decreases, frequency _______________.
Wave Equation One equation relates speed, frequency and wavelength: c =
c = Example The wavelength of the radiation which produces the yellow color of sodium vapor light is 589.0 nm. What is the frequency of this radiation?
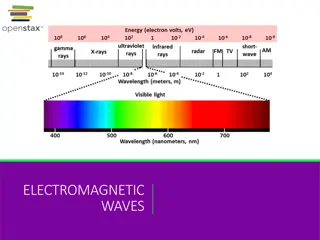
The Electromagnetic Spectrum Complete range of wavelengths and frequencies Mostly invisible
What is Color? TED Ed Video: What is color?
The Visible Spectrum Continuous spectrum: components of white light split into its colors, ROY G. BIV From 390 nm (violet) to 760 nm (red) Can be split by a prism
How do we see color? TED Ed Video: How we see color
Max Planck Particle Theory of Light Light is generated as a stream of light particles called PHOTONS Equation: E = h h =Plank s constant= 6.626 x 10-34 J s)
Example #1 (a) If the frequency of a ray of light is 5.09 x 1014 Hz, calculate the energy, in joules, of a photon emitted by an excited sodium atom. (b) Calculate the energy, in kilojoules, of a mole of excited sodium atoms.
Example #2 What is the energy of a photon from the green portion of the rainbow if it has a wavelength of 4.90 x 10-7 m?
Bohr Model of the Atom When an electron absorbs a photon of energy, the electron jumps from the ground state to an excited state Ground state lowest energy level an electron occupies Excited state temporary state when an electron is at a higher energy level
Line Spectra Pattern of lines produced by light emitted by excited atoms of an element Unique for every element Used to identify unknown elements
Niels Bohr Explanation of Line Spectra Niels Bohr Energy of an electron is quantized: can only have specific values. Energy is proportional to energy level.
Explanation of Line Spectra Electron will drop from excited state to ground state and will emit energy as a photon during the fall. Video: Atomic Emission Animation
Photoelectric Effect Nobel Prize in Physics 1921 to Einstein Occurs when light strikes the surface of a metal and electrons are ejected. Practical uses: Automatic door openers Ted Ed Video: Is Light Actually a Wave or Particle?
Conclusion Light not only has wave properties but also has particle properties. These massless particles, called photons, are packets of energy. Light has a dual nature!
Quantum Mechanics Quantum mechanics: atomic structure based on wave- like properties of the electron befo-schroedinger Schr dinger: wave equation that describes hydrogen atom
Heisenberg Uncertainty Principle The exact location and speed of an electron cannot be determined simultaneously (if we try to observe it, we interfere with the particle) You can know either the location or the velocity but not both Werner Heisenberg Electrons exist in electron clouds and not on specific rings or orbits like in the Bohr model of the atom
Quantum Numbers Quantum numbers a system of four numbers used to represent the most probable location of an electron in an atom They range from the most general locator to the most specific Analogy... state = energy level, n city = sublevel, l address = orbital, ml house number = spin, ms
1. Energy Level Principal Quantum Number: n Always a positive integer (1, 2, 3, 7) Indicates size of orbital, or how far electron is from nucleus Larger n value = larger orbital or farther distance from nucleus Similar to Bohr s energy levels or shells
n in relation to the Periodic Table n = row number on periodic table for a given element n = 1 n = 2 n = 3 n = 4 n = 5 n = 6 n = 7
2. Sublevel Angular Momentum Quantum Number: l Indicates shape of orbital Letters s, p, d, and f Energy level 1 has only sublevel s Energy level 2 has s and p Energy level 3 has s, p, and d Energy level 4-7 have s, p, d, and f
In order of increasing energy the sublevels generally go: s < p < d < f HOWEVER, there is some overlapping of sublevels at higher energy levels Ex.) 4s vs. 3d
3. Orbital The most specific piece of information is about the number and location of the electrons within the sublevel The s sublevel has 1 orbital The p sublevel has 3 orbitals The d sublevel has 5 orbitals The f sublevel has 7 orbitals Orbital - region within a sublevel where an e- can be found (homes for e-) Every orbital can hold 2 electrons!
Orbitals Orbital = electron containing area (houses for electrons) No more than 2 e- assigned to an orbital Orbitals grouped in s, p, d (and f) subshells
Shapes of Atomic Orbitals s = spherical p = peanut d = dumbbell (clover) f = flower
Capacities of levels, sublevels, and orbitals Maximum Number of Electrons in Energy Level Total Number of Orbitals Principal Energy level (n) Sublevels Present (s, p, d, or f) Number of Orbitals Present s p d f 1 2 3 4
Rules for how the electrons fill into the electron cloud: Aufbau Principle: electrons fill from the lowest energy level to the highest (they don t skip around) Pauli ExclusionPrinciple: each orbital can hold a maximum of 2 electrons at a time (and they must have opposite spins) Hund s Rule: orbitals of equal energy in a sublevel must all have 1 electron before the electrons start pairing up
Electron Configuration Definition: describes the distribution of electrons among the various orbitals in the atom Represents the most probable location of the electron! EOS
Electron Configurations The system of numbers and letters that designates the location of the electrons 3 major methods: Full electron configurations Abbreviated/Noble Gas configurations Orbital diagram configurations
Full Electron Configuration Example Notation: 1s2 2s1 (Pronounced one-s-two, two-s-one ) A. What does the coefficient mean? Principle energy level B. What does the letter mean? Type of sublevel s, p, d, or f C. What does the exponent mean? # of electrons in that sublevel
Steps to Writing Full Electron Configurations 1. Determine the total number of electrons the atom has (for neutral atoms it is equal to the atomic number for the element). Example: F atomic # = # of p+ = # of e- = 2. Fill orbitals in order of increasing energy (see Aufbau Chart). 3. Make sure the total number of electrons in the electron configuration equals the atomic number.
Aufbau Chart (Order of Energy Levels) When writing electron configurations: d sublevels are n 1 from the row they appear in f sublevels are n 2 from the row they appear in
In order of increasing energy the sublevels generally go: s < p < d < f HOWEVER, there is some overlapping of sublevels at higher energy levels Ex.) 4s vs. 3d
Writing Electron Configurations Nitrogen: Helium: Phosphorous: Rhodium: Bromine: Cerium:
Abbreviated/Noble Gas Configuration i. Where are the noble gases on the periodic table? ii. Why are the noble gases special? iii. How can we use noble gases to shorten regular electron configurations?
Abbreviated/Noble Gas Configuration Example: Tin 1. Look at the periodic table and find the noble gas in the row above where the element is. 2. Start the configuration with the symbol for that noble gas in brackets, followed by the rest of the electron configuration.
Abbreviated/Noble Gas Configuration Practice! Write Noble Gas Configurations for the following elements: Sufur: Rubidium: Bismuth: Zirconium:
Orbital Diagrams Another way of writing configurations is called an orbital diagram. (also called orbital notation) ORBITAL BOX NOTATION for He, atomic number = 2 Arrows depict electron spin 2 1 s 1s One electron has n = 1, l = 0, ml = 0, ms = + Other electron has n = 1, l = 0, ml = 0, ms = -
Orbital Diagrams Orbital diagrams use boxes (sometimes circles) to represent energy levels and orbitals. Arrows are used to represent the electrons. = orbital sublevels
Orbital Diagrams Don t forget - orbitals have a capacity of two electrons!! Two electrons in the same orbital must have opposite spins so draw the arrows pointing in opposite directions. Example: oxygen 1s22s22p4 Increasing Energy 2p 2s 1s