Understanding Electrostatics: Atomic-Level Insights into Electric Charges
Delve into the world of electrostatics to explore the study of electric charges and their interactions at the atomic level. Discover how positive and negative charges behave, how electrons and protons play a crucial role, and the conservation of charge in fundamental particles. Unravel the mysteries of static electricity and the intriguing properties of charge in matter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Electrostatics Chapter 18
Electrostatics Study of electric charges that can be collected and held in one place.
Ben Franklin
Electrostatics 1. Demonstrate how you can pick up the paper pieces without touching them in any way with your body. 2. What is occurring at the atomic level that lets you do this? Static Electricity
Electric Charge There are 2 kinds of electric charge, positive and negative. Interactions between + and explain the attraction and repulsive forces Like charges repel and unlike charges attract. Electric charge is not created or destroyed; it is conserved. Charging is the separation, not creation, of electric charges.
Microscopic View of Charge The atom has positive protons (p+) in the nucleus. The positive charge cannot move it is bound in the nucleus. The atom has negative electrons (e-) in a cloud outside the nucleus. Electrons can move from atom to atom. The atom has neutral neutrons bound in the nucleus. Ernest Rutherford p+ and e- have equal but opposite charge. JJ Thompson
Microscopic View of Charge p+ and e- have equal but opposite charge. The proton mass is much greater than the electron mass Neutral objects have an equal number of electrons and protons When neutral objects rub together, electrons may be exchanged - Objects that gain e- become negative - Objects that loose e- become positive CHARGE IS A CONSERVED QUANTITY. Charge cannot be created or destroyed. It can only be transferred. Electrons are fundamental particles. Protons and neutrons are composed of fundamental particles called Quarks
Fundamental Particles (particles with no internal structure)
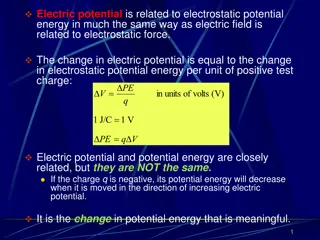
Electric Charge, q or Q Charge (like mass) is a fundamental property of matter comes in two forms which B. Franklin designated positive and negative. The electron and proton have equal amounts but opposite charge. (p+ mass is much greater than e- mass) Charge is quantized Smallest possible charge that can be easily isolated, designated e, is the magnitude of charge on 1 electron or (-e) or 1 proton (e). e is referred to as the elementary charge e = 1.602 x 10-19 C The Coulomb is the SI unit of charge
Fundamental Particles (particles with no internal structure) e is considered the elementary charge because although quarks have charge that is a fraction of e, it is not possible to stably isolate a quark
Sample Problem A certain static discharge delivers -0.5 C of electrical charge. How many electrons are in this discharge? 1 e 10 5 . 0 = 5 . 0 C Cx 19 . 1 609 x C 604 , = , 3 121 098 , 626 , 716 , , 245 e
Conductors and Insulators Charges transferred to one part of an insulator remain on that part. Insulators include glass, dry wood, plastics, and dry air Charges added to a conductor quickly spread over the surface of the object. In general, examples of conductors include graphite, metals, and matter in the plasma state
Conductors and Insulators Insulator Conductor The charges are NOT free to move around but the charge can get polarized. Many of the charges free to move around
Insulators and Conductors Conductors: silver copper gold aluminum iron steel brass bronze mercury graphite dirty water concrete Insulators: glass rubber oil asphalt fiberglass porcelain ceramic quartz (dry) cotton (dry) paper (dry) wood plastic air diamond pure water Semiconductors: Silicon Germanium carbon
Insulators and Conductors File:Krunkwerke - IMG 4515 (by-sa).jpg
Two objects are shown below. One is neutral and the other is negative. Object X will ____ object Y. a. attract b. repel c. not affect A ++ +++ -- ---
Electric Force Interaction between objects with electric charge non contact force large compared to gravity attractive or repulsive depending on charges can be analyzed using free body diagrams and Newton s laws depends on distance
Electric Force An insulating rods with small conducting spheres suspended by thin wires. Coulomb charged the spheres by conduction and measured and quantified the electric force. 1785 French physicist, Charles Coulomb
Coulombs Law Charge MAGNITUDE q k q F = 1 r 2 2 Distance between charges SI units of Force: Newton (N) SI units of Charge: Coulomb (C) 1 C is the charge on 6.24 x 1018 electrons charge of 1 e-, e = 1.6 x 10-19 C (elementary charge) k = 9.0 x 109 Nm2/C2
q q F = Coulombs Law 1 r 2 k 2 Gives the MAGNITUDE of the force between 2 charges when they are at rest. The study of charges at rest is called electrostatics. only valid for point charges (or uniformly charged spheres separated by distances much larger than the size) Coulombs law gives force on a charge due to only one other charge. If more than one charge present, Fnet is the vector sum or SUPERPOSITION of each Coulomb force.
FG Fe Interaction between objects with MASS Interaction between objects with CHARGE Attractive Attractive and Repulsive Non-contact force Non-contact force Gravitational Field g = FG/m Gm FG= Electric Field E = Fe/q q m kq 1 2 Fe= 1 2 2 r 2 r Inverse Square Law Inverse Square Law Very weak force Very strong force
Sample Problem Sphere A with charge +6 C is located 4.0cm from another sphere B with charge -3 C. What is the force of sphere B on A? 0.04m A FAB B 6 C -3 C 6 6 6 ( ) 10 )( 3 2 10 ) q q 2 x x = = 9 9 ( 10 A B F k x AB 04 . r 3 . = 101 N towards B
Sample Problem-Electric force on electron by proton: Determine the magnitude of the electric force on the electron of a hydrogen atom exerted by the single proton that is its nucleus. Assume the electron orbits the proton at its average distance of r = 0.53 x 10-10 m. Fep + e = 1.602 x 10-19 C
Sample problem Two identical positive charges separated by 12.5 cm exert a repulsive force of 1.24 N on each other. What is the magnitude of each charge? 0.125m q q F F = 1.24N
The diagrams show two charged objects and their separation. Rank the force that the left object exerts on the right object from the STRONGEST to WEAKEST force. Explain how you made the ranking. A) C > A > B B) C) + q + q + 2q + q + 1/3q + q d 2d 1/3 d ( )( d ) q q 1 1 2 ( ) q q qq = 3 2 ) FC k = = FB k FA k 2 ( 1 2 2 2 ( kq ) d d 3 2 kq 2 2 2 2 kq 3 kq 1 = = = = 6 2 d 2 2 2 4 d 2 d d 1 9