Understanding Electric Charges and Conductors in Physics
Explore the fundamentals of electrostatics, electric charges, conductors, and insulators in physics. Learn about the Law of Electric Charges, types of charge, conductors vs. insulators, and methods of charging objects through friction, conduction, and induction. Dive into the world of atoms, electrons, protons, and the role of charge in matter. Discover the different types of materials that allow or inhibit the movement of electrical charge.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
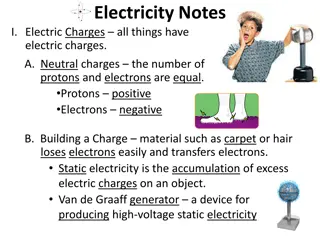
E LECT R IC CHARGE Electrostatics Electrostatics is the physics term for static charge. Electro means charge, and of course static means stationary or not moving. Charge 2 Types of Charge: Positive (+) and Negative (-) Electric Charge All matter is made up of atoms Atoms contain -Protons (+) -Neutrons (0) -Electrons (-)
Law of Electric Charges Charges with the same electrical sign repel each other, and charges with the opposite electrical signs attract each other. Protons are positively charged and electrons are negatively charged, so they are attracted to each other. Without this attraction, electrons would not be held in atoms. Unlike a gravitational force which always attracts, electrostatic force may repel or attract depending on the type charge. Ben's Rule and Paula Abdul - Opposites attract and likes repel. (+) (-) = attract (+) (+) = repel (-) (-) = repel
Conductors and Insulators -The properties of conductors and insulators are due to the structure and electrical nature of atoms. -Atoms consist of positively charged protons, negatively charged electrons, and electrically neutral neutrons. The protons and neutrons are packed tightly together in a central nucleus. - When certain types of objects are rubbed together, electrons from one object may be transferred to an object with a greater affinity for the electrons. When this happens, the object that gave up the electrons is positive, whereas the object that collected the electrons is negative.
Conductorsare materials through which charge can move freely; examples include metals (such as copper in common lamp wire), the human body, and tap water. Nonconductorsalso called insulators are materials through which charge cannot move freely; examples include rubber, plastic, glass, and chemically pure water. Semiconductorsare materials that are intermediate between conductors and insulators; examples include silicon and germanium in computer chips. Superconductors are materials that are perfect conductors, allowing charge to move without any hindrance.
How Can You Charge Objects? There are 3 ways objects can be charged: Friction Conduction Induction **In each of these, only the electrons move. The protons stay in the nucleus** Friction Charging by friction occurs when electrons are wiped from one object onto another. Example If you use a cloth to rub a plastic ruler, electrons move from the cloth to the ruler. The ruler gains electrons and the cloth loses electrons.
Conduction Charging by conduction happens when electrons move from one object to another through direct contact (touching). Example: Suppose you touch an uncharged piece of metal with a positively charged glass rod. Electrons from the metal will move to the glass rod. The metal loses electrons and becomes positively charged. Induction Charging by induction happens when charges in an uncharged object are rearranged without direct contact with a charged object. Ex. If you charge up a balloon through friction and place the balloon near pieces of paper, the charges of the paper will be rearranged and the paper will be attracted to the balloon.
Coulombs Law All bodies are able to take a charge of electricity and this is termed static electricity. The charge on a body is measure by means of the force between the charges. The Coulomb force law, which only applies to charged points, is stated below.. The force of attraction or repulsion between two charged points is directly proportional to the charges and inversely proportional to the square of the distance between them. In vector form, it is stated thus, Where; F = Force between points (N) Q1, Q2= Charges on point 1 and point 2 (Coulomb) R = radial separation on points/distance (m) = the unit vector in the direction from Q1to Q2 = Permittivity of the free space (vacuum) o Q Q = F a 1 2 12 12 2 4 R 0 12 a 12