The Phosphorus Cycle in Environmental Science
Exploring the intricate processes of the phosphorus cycle, this module delves into how solid waste, wastewater, and gaseous emissions are treated within environmental science and technology frameworks. The discussion covers the distinctive aspects of the phosphorus cycle, its impact on land and water ecosystems, and the global cycle's significance in aquatic environments. Through detailed descriptions and visuals, learners gain insights into the role of phosphorus as a limiting factor for life and its movement through various biological and geological reservoirs.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Course 3.1, Section III Phosphorus Cycle Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems Course 3.1 Environmental Science and Technology for Decision Makers Developed by AIT for Tongji University
Basic Biogeochemical Cycling 2 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Common Reservoirs 3 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
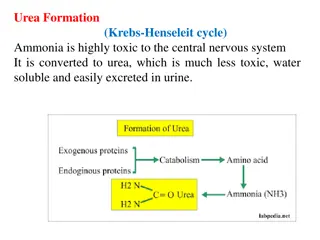
Phosphorus Cycle Distinctive Aspects of the P Cycle o No Atmospheric Component o Geologic Portion of Cycle Very Slow o Mostly involves biological transfers Phosphorus in the Earth o Most common limiting factor for life o Mostly in apatite Ca5(Cl, F)(PO4)3 Granites Phosphate Rock (recycled biological P) o Released by Weathering Mining (for fertilizer) 5 5 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle P on Land and Water Phosphorus on Land o Phosphorus in Soil o Uptake by plants o Consumption by animals o Return to soil via plant and animal waste, decay o Some lost by runoff Phosphorus in Water o Essential to aquatic life o Excess causes eutrophication Runaway productivity, excess oxygen demand o Return to water via plant and animal waste, decay o Some ends up in sediments (chitin, bone) o Sedimentary P returns to land via uplift, plate tectonics 6 6 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle: The global cycle Phosphorous often limiting in aquatic ecosystems. Levels of 0.005 mg/L may be critical to the growth of algae. No atmospheric source of phosphorous, as exists for nitrogen. Soil solution phosphorous concentrations are commonly 0.1 to 1.0 mg/L. Organic phosphorous, from dead organisms, may make up about half this amount. Although microorganisms transfer PO43- through the ecosystem, they do little to change its chemical form - oxidation state. Two pools exit -- the large, slowly cycled geologic phosphate pool, and the smaller, rapidly cycled biologically active phosphate pool 7 7 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle: The global cycle ? = ? Inorganic phosphate may be solubilized indirectly by acid forming organisms, but the biologic pool is more active. Orthophosphate appears as H3PO4, H2PO4-, HPO42-, PO43- Synthetic detergents often contain polyphosphates. Lake eutrophication, enhanced by sewage effluents, led to bans of phosphates by several state and local governments. Excess phosphorus applied in agricultural processes may move to surface waters in runoff and contribute to eutrophication. Wetlands may serve as a sink for the removal of phosphorous from wastewaters or surface waters. Phosphorous is essential to all life for its role in DNA, phospholipids (membranes) and energy transfer (ATP). 8 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle 9 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle 10 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle 11 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Phosphorus Cycle 12 12 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems
Thank You 13 Module 3: Solid waste, Wastewater and Gaseous Emissions Treatment Systems