The Model of the Atom: Discovery and Development
Explore the evolution of atomic theory from the old plumb pudding model to the groundbreaking discoveries by Ernest Rutherford and Hantaro Nagaoka. Discover how theories and experiments shaped our understanding of the atom and learn about the collaborative nature of scientific advancement. Delve into the contributions of scientists worldwide in the development of atomic physics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The Model of the Atom GCSE Physics Diversity in Science Teaching Pack
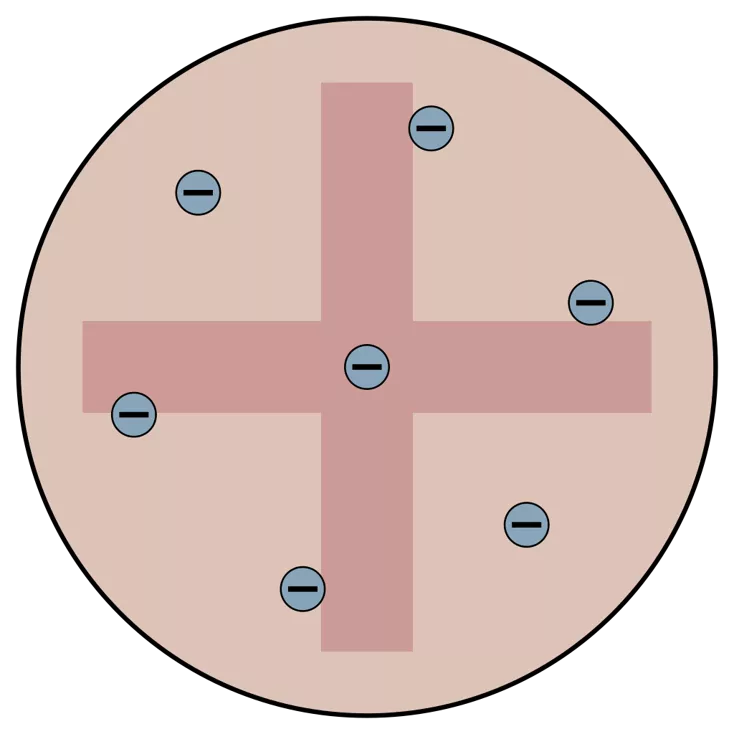
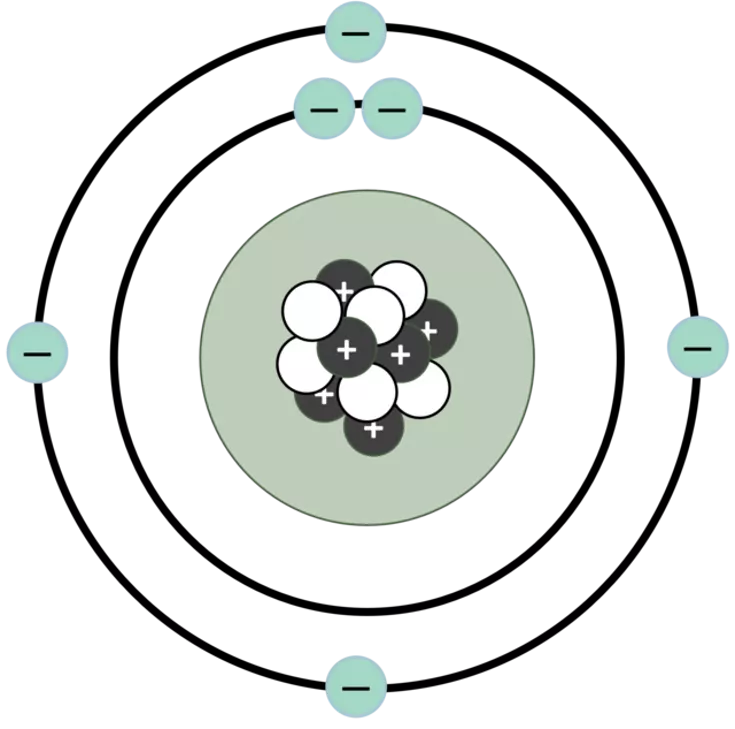
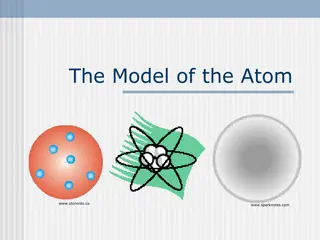
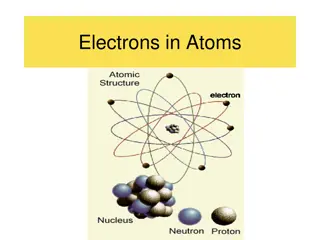
The old plumb pudding model of the atom The new model of the atom with nucleus
Who developed the new model of the atom? It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. Ernest Rutherford describing his experiment in 1909 which disproved the plumb pudding model of the atom. Ernest Rutherford (1871 1937)
But dont forget In 1904, the Japanese physicist Hantaro Nagaoka suggested a new model of the atom with a nucleus. This was five years before Rutherford did his experiment. Nagaoka called his model the Saturnian atom (like the planet with its rings!) Hantaro Nagaoka (1865 1950)
So who developed the new model of the atom? Question: How important are theories and experiments? Hantaro Nagaoka (who proposed a new theory) or Ernest Rutherford (who did an important experiment) Optional Activity
How do scientific theories and methods develop over time? Science isn t made by individuals. Scientists work together, and build on each other s ideas. Ernest Rutherford read Hantaro Nagaoka s 1904 scientific paper and cited it. Rutherford and Nagaoka also wrote letters to one another, exchanging ideas.
And whilst were here Scientists from around the world helped to develop atomic physics. Here are just a few: A picture containing person, white, black, posing A person in a lab coat A person in a suit Description automatically generated Description automatically generated with medium confidence Description automatically generated with medium confidence Wang Ganchang Dmitri Ivanenko Lise Meitner Homi J. Bhabha (China) (Ukraine) (Austria and Sweden) (India)
Specification and Licence The Model of the Atom AQA Physics Specification WS1.1 Understand how scientific methods and theories develop over time 4.4.1.1 The development of the model of the atom (common content with chemistry) GCSE Physics: Diversity in Science Teaching Pack Developed by James Poskett (University of Warwick) Licence: https://creativecommons.org/licenses/by-nc-sa/3.0/