The Metric System: A Simple Guide
The Metric System is a decimal system used for scientific measurements, with base units like meter, liter, gram, Celsius, and second. This system includes prefixes like kilo, hecto, deka, deci, centi, and milli, making conversions easy. Discover how to measure length, volume, mass, temperature, and time using tools and different units. Learn the everyday applications of millimeters, centimeters, and meters in this easy-to-understand system.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The Metric System All scientific measurements are made using the Metric System. It is also called the SI System (La Systeme International d Unites) The United States is the only major country that does not use this system.
Very Easy To Use There are base units and prefixes Based on 10 (10 and 100 and 1,000) so you multiply or divide by 10 when converting It s a decimal system Can you think of a system we use every day that is a decimal system? ___________ Hint
Metric Base Units (last names) Length Volume Mass Temp. Time (how long it is) meter (how much space it takes up) liter (for liquids) meter3 (for solids) (how much matter is in it) gram Celsius second (how much heat it has) (how long it takes)
How Do We Measure Them? (tools) Length Temperature Volume Mass Time
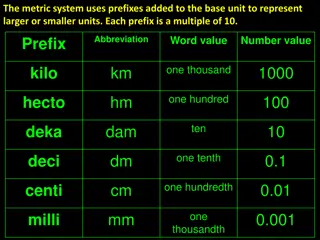
Metric Prefixes kilo hecto deka BASE UNIT 1 1000 100 10 deci centi milli 1/10 (0.1) 1/100 (0.01) 1/1000 (0.001)
Moving the Decimal for Length Larger Units, Smaller Number Smaller Units, Larger Number Base Unit kilo (km) Hecto (hm) Deka (dka) METER (m) Deci (dm) Centi (cm) Milli (mm)
Lets make it easier What s a millimeter ? About the thickness of a paperclip What s a centimeter? About the width of your pinkie finger What s a meter? Height from the floor to the door knob
The length of the yellow line in centimeters (there will be a decimal) is _______ cm The length of the yellow line in millimeters (there will not be a decimal) is _______ mm
Mass The mass is the amount of matter (or stuff) in an object and never changes unless you change the object. We use a triple beam balance to find the mass of an object in grams.
Mass vs. Weight The weight of something is the amount of gravity pulling down on an object and will change if you go somewhere with more or less gravity. Mass is NOT the same as weight!
Moving the Decimal for Mass Larger Units, Smaller Number Smaller Units, Larger Number Base Unit kilo (kg) Hecto (hg) Deka (dkg) GRAM (g) Deci (dg) Centi (cg) Milli (mg)
Use the triple beam balance to find the mass of a football in grams?
Volume The volume is the amount of space an object takes up. For liquids or oddly-shaped solids, we use a graduated cylinder or beaker to find the volume in liters (or mL).
Volume The surface of the liquid might look slightly curved. This curve is called the meniscus and we read the amount of mL from the lowest point of the meniscus. glug, glug, glug
Volume For easy-to-measure solids (like a cereal box), we use a rule to find the volume in cubic meters (m3). Volume (m3) = Length x Width x Height boom,boom,boom
Moving the Decimal for Volume Larger Units, Smaller Number Smaller Units, Larger Number Base Unit kilo (kL) Hecto (hL) Deka (dkL) LITER (L) Deci (dL) Centi (cL) Milli (mL)
Use the meniscus (lowest point of the waters surface) to find the volume of the water (there will be a decimal). The volume is about _______ mL.
Measuring Mass and Volume Take out a new piece of paper and do the following: Put your name and hour in the top right hand corner Write 1. Mass and then skip 5 lines Write 2. Volume of a liquid and then skip 5 lines Write 3. Volume of a solid and then skip 5 lines
Density Density is the amount of matter (mass) per given space (volume) Density = Mass (g) Volume (mL or cm3) For example, water s density is 1 g/mL which means that there is 1 gram of matter in every mL of water.
Density Think of it like a suitcase the more clothes you try to fit in a suitcase, the more packed it gets. More packed = More dense
Density When you compare the density of two or more objects, the denser objects will sink below the less dense objects. For example, if an object has a density of 2 g/mL what will it do in water? What if an object has a density of 0.2 g/mL?
Density EXAMPLE OF SAME MASS (151 g): Spongebob Mac & Cheese Easter jelly beans EXAMPLE OF SAME VOLUME (5,452 cm3): Red Swirly bowling ball Spider Web bowling ball
Density REMEMBER: Denser objects sink below less dense objects! Number your paper 1 5 Order the following objects from least dense (#1) to most dense (#5): Water (blue), Syrup, Oil, Rubbing Alcohol (green), Oil, and Dish Soap