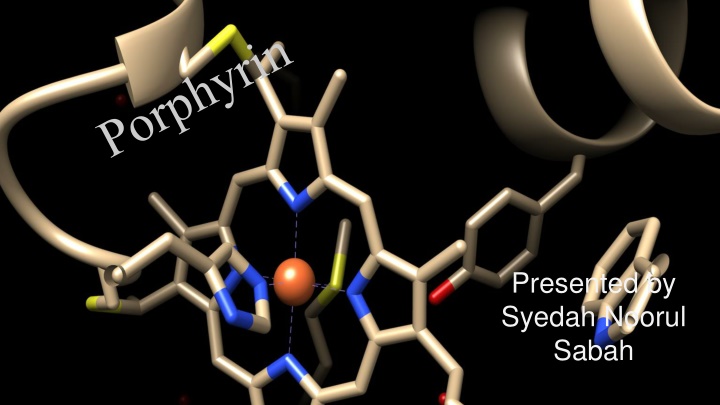
The Importance of Porphyrins in Living Organisms
Porphyrins are essential macrocyclic compounds found in various biologically important molecules, playing a crucial role in the metabolism of living organisms. Derived from the Greek word "Porphura," meaning purple, porphyrins are organic pigments with a distinct porphine macrocycle structure. They can bind metal ions to form metalloporphyrins and exhibit aromatic properties, undergoing various substitution reactions. Solubility, reactivity, and tautomeric preferences characterize these molecules, shedding light on their biological significance.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Presented by Syedah Noorul Sabah
Introduction Porphyrins are an important class of naturally occurring macrocyclic compounds found in various biologically important compounds that play a very important role in metabolism of living organisms. The word Porphyrin is derived from the Greek word Porphura meaning purple. They are actually organic pigments, of both natural and synthetic origin, all of which contain the porphyrin ring. The common feature of all these molecules is the basic structure of the porphine macrocycle, which consists of a 16-atoms ring containing four nitrogen atoms,obtained by linking four tetrapyrrolic subunits with four methine bridge.
Figure 1.The porphyrin. Pyrrole rings are shown in red and methine bridges in green.
Numbering of Porphyrin molecule Porphyrins are numbered along the peripheral carbon atoms as shown in fig. The inner nitrogens are given locants 21-24. Figure 2. Numbering of the porphyrin molecule. The positions 5,10,15 and 20 are called meso and 2,3,7,8,12,13,17 &18 are called beta pyrole positions
Porphyrins are generally substituted on the periphery. There are 12 positions that can be substituted. There are eight positions on the pyrrolic rings and four methine bridges correspond to meso positions. The unsubstituted porphyrin has been named porphine. Naturally occurring porphyrins are generally beta-substituted in an unsymmetrical fashion. Protoporphyrin IX Figure 3
Properties The porphyrin macrocycle is a highly-conjugated molecule containing 22-electrons, but only 18 of them are delocalized according to [18]annulene model, proposed by E. Vogel. According to that model, a delocalization pathway is distinguished in the macrocycle, as shown in Figure 4, which is aromatic in the traditional H ckel sense.The porphyrin is thus viewed as a bridged diaza[18]annulene.
The size of the macrocycle is perfect to bind almost all metal ions and indeed a number of metals (e.g. Fe, Zn, Cu, Ni, and Co) can be inserted in the center of the macrocycle forming metalloporphyrins. Being aromatic, porphyrins undergo electrophilic substitution reactions like halogenation, nitration, sulphonation, acylation, deuteration, formylation etc at both meso and beta positions . However,the reactivity at two sites is different. Solubility of porphyrins in water or other solvents is controlled by the nature of substituents at beta or meso positions. Porphyrins are weak bases and can be protonated to form dications. In the unprotonated porphyrin (called free base) the two inner protons are mobile and jump freely among the four nitrogens. The trans (21,23-H) tautomer, shown in all schemes, is energetically preferred to the cis form (21,22-H).
Porphyrins form a great number of complexes with metal ions and some nonmetals .The coordinating environment provided by porphyrins is very flexible and can be fine-tuned to particular oxidation and spin states by varying peripheral substitution and axial ligands. This tunability was probably Nature's reason to choose porphyrin as its "workhorse macrocycle." Of the naturally occurring metalloporphyrins the iron complexes, called hemes, are by far the most important. They make the reactive centers of numerous heme proteins, responsible for oxygen transport and storage (hemo- and myoglobin), electron transfer (cytochromes), and oxidation of organic substrates (oxygenases of the P450 family).
Uses of Porphyrins Because of the unique chemistry of porphyrins, they are able to serve in several ways As a metal binder (ligands) As a solar cell (convert light or chemical energy) As an oxygen transport medium (hemoglobin) As an electron transfer medium (conducting polymers) Gene regulation Drug metabolism Iron metabolism Hormone synthesis
Spectral studies of Porphyrins Uv-vis spectra of porphyrins Porphyrins being highly conjugated -electron systems show characteristic absorption bands in UV-Vis region of em-spectrum. It has been found that UV-visible spectra of porphyrins consist of two distinct regions: in the near ultraviolet and in the visible region. UV-vis spectrum of porphyrin
Colour of porphyrins is due to aborptions within porphyrin rings involving the excitation of electrons from to * porphyrin ring orbitals. The electronic absorption spectrum of a typical porphyrin consists of a strong transition to the second excited state (S0 S2) between 380-500 nm depending on whether the porphyrin is beta or meso substituted(the Soret or B band) and a weak transition to the first excited state (S0 S1) between 500-750 nm (the Q band). Internal conversion from S2to S1is rapid so fluorescence is only detected from S1. UV spectrum of Porphyrins depends upon: a) Conjugation pathway b) Symmetry of porphyrin The B and the Q bands both arise from * transitions and can be explained by considering the four frontier orbitals (HOMO and LUMO orbitals) (the Gouterman four orbital model)
Gouterman Four-Orbital Model Martin Gouterman in 1960 proposed 4 orbital model to explain the absorption spectrum of porphyrins.According to this theory, absorption bands in porphyrins arise due to transitions between 2 HOMO s and 2 LUMO s. The energy difference between Homo, and Lumo s can be altered by changing the central atom or the substituents on the ring. The HOMOs were calculated to be an a1u and an a2u orbital, while the LUMOs were calculated to be a degenerate set of eg orbitals. Representation of the four Gouterman orbitals in porphyrins.
Transition between these two orbitals gives rise to two excited states.Orbital mixing splits these two states in energy, creating a higher energy state with greater oscillator strength, giving rise to the Soret band almost 10 times more, and a lower energy state with less oscillator strength, giving rise to the Q-bands. The electronic absorption spectrum of a typical porphyrin consists therefore of two distinct regions. Drawing of the energy levels of the four Gouterman orbitals upon symmetry lowering from D4h to C2V. The set of eg orbitals gives rise to Q and B bands
LUMO HOMO Orbital diagrams showing possible transitions for porphyrins. Note that while the HOMOs are shown to be degenerate in both cases, the actual relative energies will depend on the substitution of the rings.
It has been found that the absorption spectrum of porphyrins shows a significant change by: Changing/Insertion of metal atom Protonation of two of inner nitrogen atoms However the variations in the peripheral substituents on the ring leads to minor changes in visible absorption spectrum.
H1 Nmr spectra of porphyrins The large aromatic ring current shifts produced by the circulating pi-electrons of the porphyrin macrocycle dominate the HNMR spectra of the porphyrins.An aromatic compound, having a closed loop of electrons, can sustain an induced ring current; protons on the ring are deshielded while protons above and below the ring are shielded. . Since porphyrins contain 3-different types of H- nuclei ,nmr spectra of porphyrin generally gives 3-peaks a) 4-meso H b) 8-beta H c) NH proton
1Hmr spectra of *Hmr spectra (220 MH z pulse FT ) of (a) porphin (1), saturated solution (~5 X 10~ 5 M) in CDCl3, 2000 pulses, repetition rate 0.49 sec, spectrum width 4500 Hz;
The chemical shift of NH protons, much like OH protons, can occur virtually anywhere in 1H NMR spectra. The Hvalue not only depends on the exact molecular structure of a compound, but also on the solvent used, the concentration, the temperature, the acidity, the presence of hydrogen-bonding,aromaticity, etc. Protons attached to nitrogen may be exchangeable with 5deuterium if a protic deuterated solvent (e.g., D 2O, CD3OD) is used, and the NH peak may diminish, or disappear
At room temperature , the N H exchange between the possible tautomeric hydrogens forms is fast on the n.m.r. time scale and only one resonance is observed for each group of protons. At low temperatures the beta -pyrrole proton signal is split. IR - spectra: The spectral bands at 3314 and 966 cm 1 in the IR spectrum of porphyrin ligand are due to N H stretching and bending vibrations of the porphyrin ligand core, respectively.
https://www.researchgate.net/profile/Nathir_Al- Rawashdeh/publication/267459858_MACRO_TO_NANO_SPECTROSCOPY_Macro_to_Nano_Spectroscopy_Edited_by_Jamal_Uddin_P ublishing_Process_Manager_Marina_Jozipovic_Technical_Editor_Teodora_Smiljanic_Cover_Designer_InTech_Design_Team/links/54509 4c50cf24e8f7374bd43/MACRO-TO-NANO-SPECTROSCOPY-Macro-to-Nano-Spectroscopy-Edited-by-Jamal-Uddin-Publishing-Process- Manager-Marina-Jozipovic-Technical-Editor-Teodora-Smiljanic-Cover-Designer-InTech-Design-Team.pdf#page=9 https://www.cpp.edu/~lsstarkey/courses/CHM-Lab/PorphyrinBasics.pdf http://www1.lasalle.edu/~prushan/Abs%20and%20Fluor%20of%20TPPH2.pdf https://core.ac.uk/download/pdf/12163457.pdf http://www1.lasalle.edu/~prushan/Abs%20and%20Fluor%20of%20TPPH2.pdf https://epub.ub.uni-muenchen.de/2549/1/2549.pdf