The Carnot Cycle in Thermodynamics
Explore the principles of the Carnot cycle, efficiency of a Carnot engine, and the significance of isotherms and adiabats in thermodynamics. Gain insights into the working of such engines and their efficiency calculations through a series of informative visual representations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
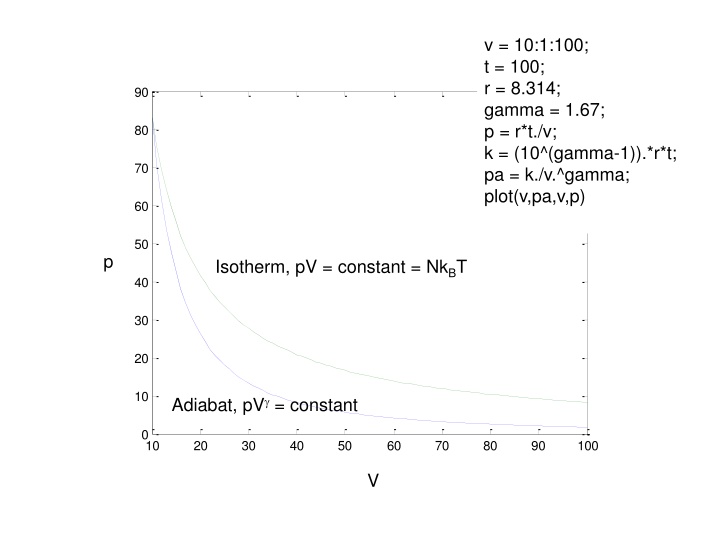
v = 10:1:100; t = 100; r = 8.314; gamma = 1.67; p = r*t./v; k = (10^(gamma-1)).*r*t; pa = k./v.^gamma; plot(v,pa,v,p) 90 80 70 60 50 p Isotherm, pV = constant = NkBT 40 30 20 10 Adiabat, pV = constant 0 10 20 30 40 50 60 70 80 90 100 V
v = 10:1:100; th = 100; tl = 50; r = 8.314; gamma = 1.67; p1 = r*th./v; k1 = (30^(gamma-1)).*r*th; pa1 = k1./v.^gamma; p2 = r*tl./v; k2 = (30^(gamma-1)).*r*tl; pa2 = k2./v.^gamma; plot(v,p1,v,pa1,v,p2,v,pa2) Carnot cycle
Carnot cycle pA, VA, TA Isotherm 1 Th p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Isotherm 2 pC, VC, TC V
Carnot cycle pA, VA, TA Isotherm 1 Th p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Isotherm 2 pC, VC, TC V
Carnot cycle pA, VA, TA Isotherm 1 Tl p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Ql Isotherm 2 pC, VC, TC V
Carnot cycle pA, VA, TA Isotherm 1 Tl p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Ql Isotherm 2 pC, VC, TC V
Why such a strange engine? Will discuss in class
Efficiency of a Carnot engine ??,??,?? Isotherm 1 ??= ??= ? p ??,??,?? Adiabat 2 Adiabat 1 ??,??,?? ??,??,?? Isotherm 2 ??= ??= ?? V
Efficiency of a Carnot engine ??,??,? Isotherm 1 ? = ?? ln?? p ?? Adiabat 2 ??,??,? ? = 0 ???? ? 1= ? ?? ? 1 Adiabat 1 ? = 0 ? ?? ? 1= ???? ? 1 ??,??,?? ??,??,?? Isotherm 2 ??= ???ln?? ?? V
Efficiency of a Carnot engine ? = ?? ln?? (1) From isotherm 1 ?? ? 1= ???? ? 1 (2) From adiabat 1 ? ?? ??= ???ln?? (3) From isotherm 2 ?? ? 1= ? ?? ? 1 ???? (4) From adiabat 2 From the first law of thermodynamics: ? = ? + ? For the complete Carnot cycle ? = 0 since ? is a state variable
Efficiency of a Carnot engine ? = ?? ln?? (1) From isotherm 1 ?? ? 1= ???? ? 1 (2) From adiabat 1 ? ?? ??= ???ln?? (3) From isotherm 2 ?? ? 1= ? ?? ? 1 ???? (4) From adiabat 2 From the first law of thermodynamics: ? = ? + ? For the complete Carnot cycle ? = 0 since ? is a state variable ? = ? ?is the work done on the engine (system), let ? be the work done by the engine ? = ? = ? From (1) and (3): ? = ? + ??= ?? ln?? ??+ ???ln?? ??
Efficiency of a Carnot engine ? = ?? ln?? (1) From isotherm 1 ?? ? 1= ???? ? 1 (2) From adiabat 1 ? ?? ??= ???ln?? (3) From isotherm 2 ?? ? 1= ? ?? ? 1 ???? (4) From adiabat 2 From (1) and (3): ? = ? + ??= ?? ln?? ??+ ???ln?? ??= ? Efficiency is defined as:Output Input Output is the work done by the engine i.e. ? and input is the heat absorbed by the engine i.e. ? ? ? ? = ? + ?? = 1 + ?? ? efficiency = ?
Efficiency of a Carnot engine ? = ?? ln?? (1) From isotherm 1 ?? ? 1= ???? ? 1 (2) From adiabat 1 ? ?? ??= ???ln?? (3) From isotherm 2 ?? ? 1= ? ?? ? 1 ???? (4) From adiabat 2 = ? + ?? ? ? ? = 1 + ?? ? efficiency = ? ???ln?? ln?? = 1 ?? ?? ?? (from (1) and (3)) ? = 1 + ?? ln?? ln?? ? ?? ?? ? 1 ? 1 ?? =?? ?? =?? (2) ? ?? ?? ? ?? ?? and (4) = = ?? ?? ?? ?? ?? ??
Efficiency of a Carnot engine ? = 1 ?? ?
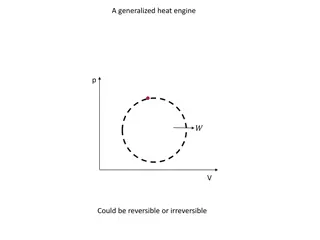
Laws of thermodynamics 0. There is a game 1.You can never win 2. You cannot break even, either 3. You cannot quit the game
Carnot engine: Schematic representation ? ? = ? ? = ? + ??= ? ?? Carnot ??= ?? = ?? ??
Carnot engine: Schematic representation ? ? ? = ? ?? Carnot ?? ??
Carnot engine is reversible ? ? ? = ? ?? Carnot ?? ??
Carnot engine is reversible (refrigerator) ? ? ? = ? ?? Carnot ?? ??
Carnots theorem reversible Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine
Carnot engine is reversible (refrigerator) ? ? ? ?? ? = ? ? = ? ?? R Carnot ?? ?? ?? Adjust the cycles so that ? = ?
Carnot engine is reversible (refrigerator) ? ? ? ?? ? = ? ??= ? R Carnot ?? ?? ??
Carnot engine is reversible (refrigerator) ? ? ? If ? > ? then: ? ? >? ?? ? ? = ? ??= ? R Carnot < ? ? ? > 0 ? ?? Also: ? ??= ? ?? ?? = ?? ?? > 0 ? ? ??
Is this possible? ? ? ? ? ? = ?? ?? > 0 ? ? ?? ? = ? ??= ? R Carnot ??? ??> ?? ? > ? ?? ?? ?? ?? ??
The Second Law of Thermodynamics Clausius statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body. ? ?
Carnots theorem reversible Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine ????< ???? ????< ????
For reversible engines ? ? ? ?? ? = ? ??= ? R Carnot ?? ?? ?? ? ? ??? ? ? ? = ?
Carnots theorem Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine All reversible engines working between two temperatures have the same efficiency as ?Carnot
The Second Law of Thermodynamics Clausius statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body. Kelvin-Planck statement: It is impossible to construct a device that operates in a cycle and produces no other effect than the performance of work and the exchange of heat from a single reservoir.
Carnot refrigerator and Kelvin violator ? ? ? ? ??= ? = ??> 0 = ? ?? ? ? ? = ? Kelvin violator Carnot ?? ??
Carnot engine and Claussius violator ? ? ?? ? = ? ?? Claussius violator Carnot ?? ?? ??