Carnot Cycle and Multi-Source Engine Characteristics
Carnot cycle is a thermodynamic process converting thermal energy into work, while a multi-source engine offers high efficiency, better reliability, and easier maintenance. It can run on various heat sources and is safe, modular, and flexible. The engine's mechanisms are simpler and suitable for low temperatures. Additionally, it is safe, discrete, and oxygen-free with low explosion risks and independent operation capability without an air supply. The PV diagram describes pressure-volume changes, indicating efficiency improvements. The engine's modularity allows for different applications with minor modifications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
characteristics High Effic iency Multi-Source Engine Better reliabil ity and eas ier maintenance Reversible Safe, discrete and oxyge n-free Modularity and flex ibility
Multi-Source Engine Carnot engines can run directly on any available heat source.
r Bette r reliability and easier maintenance The engine mechanisms are in some ways s impier than other reciprocating engine type. No valves are needed, and the burner system can be relative ly s imple. Unlike other technologies, the Carnot engine is very suitable for low temperature.
Carnot cycle The Carnot cycle , is a thermodynamic process, that describes how a fluid is used to convert ther mal energy into wor k. Nicolas It Leonard Sadi carnet. is related to the theory of heat engines.
Safe, discrete and oxygen- free A Carnot engine: uses a single-phase working f luid thus for a properly designed syste m the risk of explosion is low. In comparison, a steam eng ine uses a two phase gas/ liquid working f luid, so a faulty release valve can cause an explosion. Moreove r the Carnot engine can be built to run quietly and without an air supply, for air independent operation (i.e: submarines).
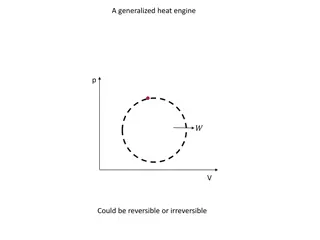
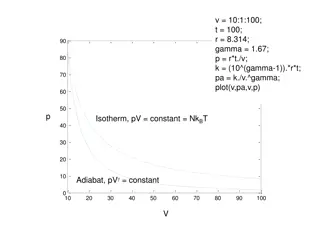
P-V diagram is used to describe A pres,sure volume diagram correspondi ng changes in volu1 me and system. The called an indicator d iagram, was pressure in a PV diagram, developed by James Watt and his employee John Southe rn (1 75 8-181 5) to improve the efficiency of engines.
Modularity and flexibility Possibility to use the same engine for different application s with only minor modifications. Moreover, the Carnot architecture allows to deve lop a wide range of same design. It means the scaling up or down t he engine power without the need of cost ly and time consuming design studies. power based on the possibility of
PV diagram This is the PV diagram. n -b lsothennal expansion TL T,, /> r- ' I I I I ' d- Adiabatic compression I I ' n I\\ I '''...... --- - - ' H o-o Q = O 0 c -d lsothemtitl oon1prcss-ion
Cont.. isothermalsegments (AB and CD) occur whe n the re is perfect thermal contact between the wor king fluid and one of the reservoirs, so that whateve r heat is needed to maintain constant temperature wil l flow into or out of the wor king fluid, from or to the reservo ir. A a d i a b a t i c Vob xn e
Cont.. the adiabatic segme nts (BC and DA) occur whe n the re is perfect the rmal insulation between the wor king fluid and the rest of the universe, including both reservoirs, thereby preventing the flow of any heat into or out of the wor king fluid. A n '--:-.:- i s O'therra.a.l = = - C Voh xn e
Sequence of operation Phases of the Carnot's Cyc le The sequence of steps Reversible isothermal ex pans ion (1 -2 , TH=constant) Reversible adiabatic expans ion (2 -3, Q=O, TH-?TL) Reversible isothermal compress ion (3-4, TL=constant) Reversible adiabatic compression (4- 1, Q=O, TL-?TH) in the Carnot's
1 sequence IsotherlnlI t Adl1bltic comJ)feSSlon J Adl b ltlc nslon I sothermal comll'95Slon nslon Cold ... . 111111, Tc
Thermal efficiency In thermodynamics, the the rmal effic iency a measure of perfor mance of a device that uses thermal energy. For example: an internal combustion engine, a steam turbine or a steam engine, a boiler, a furnace, or a refrigerator for example. is
Cnt.... The Carnot Cyc le is an entirely theoret ical thermodynami c cyc le utilizing reversible processes. The ultimate thermaleffic ie ncy can the n be used to compare the efficienc ies of other cycles operat ing betwee n the res. same two temperatu
Cnt.. The thermal effic iency of any engine wor king between the tempe ratures of i.e. increase the temperatu re differenc e under which the engi ne work s. T, and 72 is :
Applications 1) Al I types of ve hic les that we use, cars, motorcycles, trucks, ships, aero planes, and many other types wor k on the basis of second law of thermodynamics and Carnot Cyc le. They may be using petrol engine or diesel engine, but the law remains the same. I I ( 1-1 1 1 1- - - N t v... . -
Applications 2) All the refrigerators, deep freezers, indust rial refrigeration systems, alI types of air-conditioning systems , heat pumps, etc work on the ba.sis of the Carnot cycle. B sicHeat PumpConfiguration ........ I - ! - l.'M-81" ' - I &.:.- -
Application Thermocouple Lead wire ....--:-+-- ,..o....- 1Vout Gage I + +1I A B I Target surface Ice bath (kno"" constant temperature lor reference)