The Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The mechanism involves the formation of an activated carbonyl and subsequent rearrangement with driving forces like increased cation stability and ring strain relief. Learn more about this reaction and its historical significance in this comprehensive overview.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The Prins-Pinacol Reaction Organic Pedagogical Electronic Network Andy Clevenger University of Utah
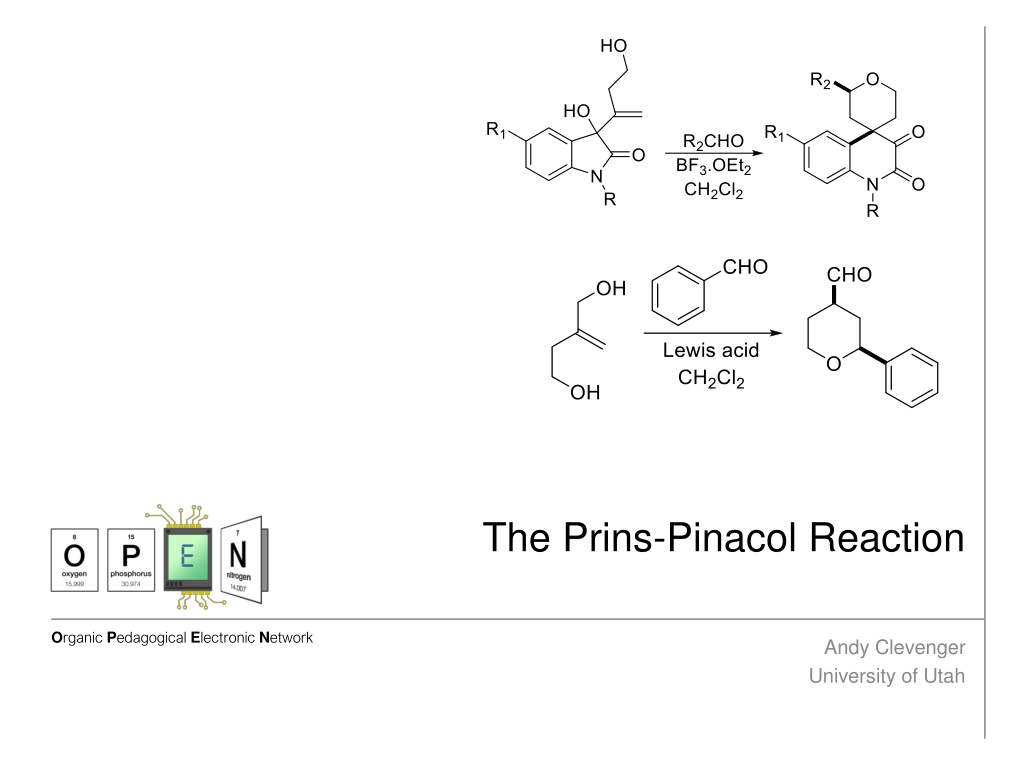
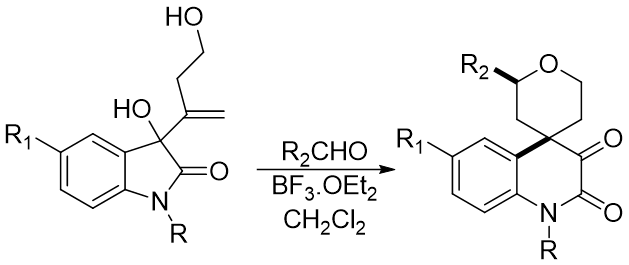
Overview The Prins-Pinacol reaction is a two step process. It begins with the Prins reaction, which is the attack by a nucleophilic alkene on a Lewis acid-activated aldehyde. This forms a cationic intermediate. The pinacol rearrangement is a methyl shift which pushes the cation on to an oxygen, which is then deprotonated. (top) Reddy, B.V.S.; Reddy, S.G.; Reddy, M.R.; Bhadra, M.P.; Sarma, A.V.S. Org. Biomol. Chem. 2014, 12, 7257-7260 (bottom) Cloninger, M.J.; Overman, L.E. J. Am. Chem. Soc. 1999 121, 1092-1093
History In 1919, Dutch chemist Hendrick Jacobus Prins found the first step of this reaction by combining styrene and formaldehyde in concentrated H2SO4. The pinacol rearrangement was first found by Wilhelm Rudolph Fittig in 1860. It is an acid-catalyzed method for converting a 1,2-diol into a carbonyl. Prins: https://en.wikipedia.org/wiki/Prins_reaction Pinacol: https://en.wikipedia.org/wiki/Pinacol_rearrangement
Prins-Pinacol Mechanism: Forming the Activated Carbonyl In this first step, the more exposed alcohol attacks the activated aldehyde. The oxygen on the activated aldehyde is removed as the Lewis Acid complex and a highly reactive oxocarbenium ion is formed. Reddy, B.V.S.; Reddy, S.G.; Durgaprasad, M.; Bhadra, M.P.; Sridhar, B. Org. Biomol. Chem.2015, 13, 8729-8733.
Prins-Pinacol Mechanism: Prins Reaction The alkene attacks the activated carbonyl and forms the six-membered ring in the Prins reaction. The cation is now at a more stable tertiary center. Reddy, B.V.S.; Reddy, S.G.; Durgaprasad, M.; Bhadra, M.P.; Sridhar, B. Org. Biomol. Chem.2015, 13, 8729- 8733.
Prins-Pinacol Mechanism: Pinacol Rearrangement In this pinacol rearrangement, there is an aryl methyl shift. The C-C bond shifts to the carbocation position while one of the lone pairs on the alcohol closes down to form a carbonyl. With deprotonation, the Prins- Pinacol reaction is complete. Reddy, B.V.S.; Reddy, S.G.; Durgaprasad, M.; Bhadra, M.P.; Sridhar, B. Org. Biomol. Chem.2015, 13, 8729- 8733.
Driving Forces Behind the Prins-Pinacol Reaction Most of the force driving this reaction to completion is the increased stability of the cation. Ring strain relief can also drive this reaction to completion. In this example, the strained four membered ring reacts and becomes a fused pyran-cyclopentanone compound which has much less ring strain. Reddy, B.V.S.; Reddy, S.G.; Reddy, M.R.; Bhadra, M.P.; Sarma, A.V.S. Org. Biomol. Chem. 2014, 12, 7257- 7260
Prins-Pinacol Applications Complex backbones of natural products Stereoselective tetrahydropyran synthesis Many natural product syntheses Convienent reaction pathway to establish a carbonyl and close a ring system
Questions Why can t the Prins-Pinacol reaction occur on a ketone instead of an aldehyde? A) Steric hindrance-the alkene can t attack the oxocarbenium ion B) The oxocarbenium ion will not be able to form C) The carbonyl will not be activated by the Lewis acid D) The alkene can t attack the ketone oxocarbenium ion due to reduced reactivity
Questions What is the role of the Lewis acid in the Prins-Pinacol reaction? A) It stabilizes the oxocarbenium ion B) It activates the aldehyde for attack by an alcohol C) It stabilizes the carbocation intermediate D) It deprotonates the protonated carbonyl at the end of the reaction
Questions Which of these compounds is the correct Prins-Pinacol product for the given reaction? A) C) B)
Questions What can be a driving force behind the Prins-Pinacol reaction? A) Ring strain relief B) Increased carbocation stability C) Increased stability with the formation of a carbonyl D) All of the above
Questions What functional group can undergo the Prins-Pinacol reaction with an aldehyde? A) A 1,2-diol B) A 1,2-diketone C) An alpha, gamma-hydroxyl alkene D) An ester
Contributed by: Andy Clevenger (Undergraduate) University of Utah, 2016 This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License.