Overview of Group 16 P-Block Elements
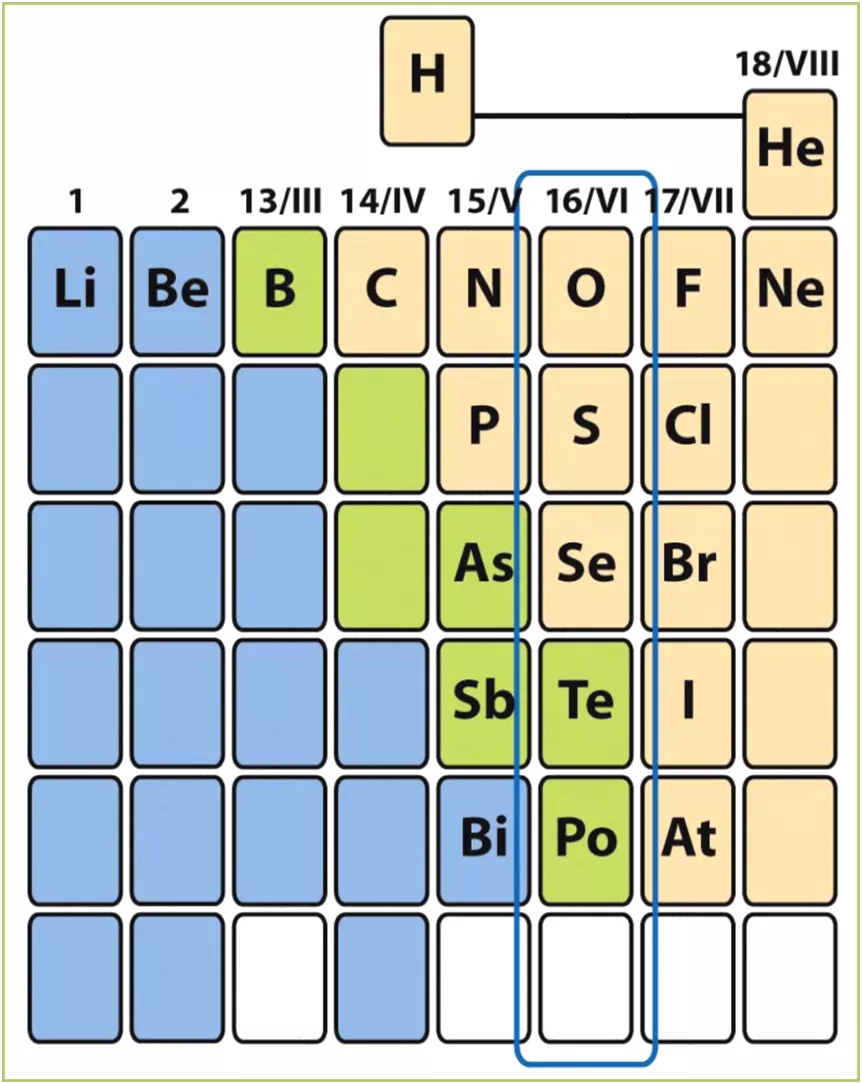
Group 16 P-Block Elements, also known as Group VIA or Chalcogens, include oxygen, sulfur, selenium, tellurium, and polonium. These elements exhibit varying properties from non-metallic to semi-metallic to metallic. The group shows a general trend of increasing metallic properties down the group, along with changes in melting points, boiling points, densities, and oxidation states. Their reactivity with hydrogen and acidic nature are also distinctive features. This article provides a comprehensive overview of the characteristics and behavior of Group 16 elements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
P-block Elements VI A- Group 16 Dr. Nouf H. Alotaibi
P-block Elements- VI A elements It consists of the elements oxygen, sulfur, selenium, tellurium and polonium. Electron configurations ns2np4 (n is the period number). Oxygen is diatomic gas while sulphur, selenium and tellurium are octa atomic S8, Se8 and Te8 molecules, which have a ring structure. Oxygen and sulfur are most definitely non-metals. Elements become increasingly more nonmetallic toward the right- hand side of the periodic table. Selenium and tellurium both possess some semimetallic behaviour. Polonium exhibit metallic character. O and S have the most significant chemistry in this group. Oxygen is the more reactive.
P-block Elements- VI A elements General Trend The metallic properties increase down the group. Element M.p ( C) B.p( C) O2 -219 -183 S8 119 445 Se8 221 685 Te 452 987 Po 254 962 The melting and boiling points show the rising trend characteristic of nonmetals followed by the drop at metallic polonium. Density, atomic radius, and ionic radius all increase down the group. Ionization energy decreases down the group.
P-block Elements- VI A elements Oxidation State The most common oxidation state is -2; Sulfur can exist at a +4 and +6 state, For Se, Te, and Po, oxidation states of +2, +4, and +6 are possible. The even-numbered oxidation states from +6, through +4 and +2, to -2. The stability of the -2 and +6 oxidation states decreases down the group. whereas that of the +4 state increases. The stability of -2 oxidation state decreases down the group due to increase in atomic size and decrease in electronegativity. The stability of +6 oxidation state decreases and +4 oxidation state increases due to inert pair effect. Oxygen does not show +6 oxidation state due to absence of d orbitals.
P-block Elements- VI A elements Reactivity with hydrogen: All group 16 elements form hydrides of type MH2. Group 16 hydrides have a bent shape. Hydride bond angles decrease and bond lengths increase down the group. Order of bond angle H2O > H2S > H2Se > H2Te > H2Po The HMH bond angle in water is 104 .311 but in other hydrides it is almost equal to 90 . In H2O oxygen is involved in sp3 hybridisation but in other hydride pure p orbitals are participated in bonding. Oxygen and sulphur form less stable polyoxides and polysulphides like H2O2, H2S2, H2Sn,(n=2 to10). H2O and H2O2 can form H bonds, and therefore have higher melting and boiling points than other H2M compounds.
P-block Elements- VI A elements Reactivity with hydrogen: Acidic nature: H2O < H2S < H2Se < H2Te This is because the H-E bond length increases down the group. Therefore, the bond dissociation energy down the group. H2O is a liquid while the hydrides of the other VIA elements are a gas. (poisonous gases) This is because strong hydrogen bonding is present in water. This is due to small size and high electronegativity of O.
P-block Elements- VI A elements Reactivity with oxygen: The other elements in the group are oxidized by O2: M(s) + O2 (g) MO2 (M = S, Se, Te, Po) VI A group elements form two types of oxides; dioxides of the type MO2 and trioxides of the type MO3. Acidity also decreases down the group. SO2 is a gas whereas SeO2 is solid. This is because SeO2 has a chain polymeric structure whereas SO2 forms isolated units. SO2 is oxidized further, and the product is used in the final step of H2SO4 manufacture. 2 SO2 (g) + O2 (g) 2 SO3 (g)
P-block Elements- VI A elements Reactivity with oxygen: Types of oxides: Acidic oxides: Non- metallic oxides are usually acidic in nature. SO2 + H2O Basic oxides: Metallic oxides are mostly basic in nature. H2SO3 (sulphurous acid) Basic oxides dissolve in water forming bases Na2O + H2O Amphoteric oxides: They show characteristics of both acidic as well as basic oxides. 2 NaOH Al2O3 + 6 HCl(aq) Al2O3 + 6 NaOH(aq) + 3 H2O(l) Neutral oxides: These oxides are neither acidic nor basic. Example: CO, NO and N2O 2 AlCl3(aq) + 3 H2O 2 Na3 [Al(OH)6](aq)
P-block Elements- VI A elements Reactivity with halogens: Halides are formed by direct combination: M(s) + X2 (g) various halides (M = S, Se, Te ; X = F, Cl) VI A group elements form; monohalides of the type M2X2; dihalides of the type MX2; tetrahalides of the type MX4; and hexahalides of the type MX6 (Where M = S, Se, Te ; X = halogen). The oxidation states of S, Se and Te in monohalides is +1, in dihalides is +2, in tetrahalides is +4 and in hexahalides is + 6. Hexafluorides are only stable halides which are gaseous and have sp3d2 hybridisation and octahedral structure.
P-block Elements- VI A elements Reactivity with halogens: Their structure and reactivity patterns depend on the sizes of the central atom and the surrounding halogens. As the central atom becomes larger, the halides become more stable. This pattern is related to the effect of electron repulsions due to crowding of lone pairs and halogen atoms around the central atom. This is opposite to the previously observed bonding patterns, where bond strength decreases as bond length increases. The stability of halides decreases in the order F- > Cl-> Br- > I- . This is because M-X bond length increases with increase in size.
P-block Elements- VI A elements Allotropes in the Oxygen Family Oxygen has two allotropes: O2, which is essential to life, and O3 or ozone, which is poisonous. Sulfur has more than 10 different forms, due to the ability of S to catenate. S S bond lengths and bond angles may vary greatly. For example: a. Yellow Rhombic ( - sulphur) and b. Monoclinic ( - sulphur): 369 K Sulphur Sulphur At 369 K both forms are stable. It is called transition temperature. Both of them have S8 molecules. The ring is puckered and has a crown shape. Selenium has several allotropes, some consisting of crown-shaped Se8 molecules.
P-block Elements- VI A elements Oxygen Oxygen is a gas at room temperature and 1 atm. It is colorless and tasteless. It is the most abundant element in the Earth's crust and the most abundant element in sea water. It is second to nitrogen as the most abundant in the atmosphere. There are many commercial uses for oxygen gas. It is used in the manufacture of iron, steel, and other chemical manufacturing. It is also used in water treatment, as an oxidizer in rocket fuel, for medicinal purposes, and in petroleum refining. It exists as two allotropes: O2 and O3. Its primary oxidation states are -2, -1, 0 and -1/2 in O2- .
P-block Elements- VI A elements Oxygen When oxygen reacts with metals, it forms oxides that are mostly ionic in nature. It is rarely featured as the central atom in a structure and can never have more than 4 elements bonded to it due to its small size and its inability to create an expanded valence shell. When it reacts with hydrogen, it forms water, which is extensively hydrogen-bonded, has a large dipole moment and is considered an universal solvent.
P-block Elements- VI A elements Sulfur Sulfur is a solid at room temperature and 1 atm pressure. It is the sixteenth most abundant element in Earth's crust. It exists in a variety of forms naturally, including elemental sulfur, sulfides, sulfates, and organosulfur compounds. Sulfur is very unique in its ability to form a wide range of allotropes, more than any other element in the periodic table. At room temperature, the sulfur molecule is a crown-shaped ring of eight atoms. The most stable S allotrope is orthorhombic -S8, which consists of cyclo-S8.
P-block Elements- VI A elements Sulfur The most common state for sulfur to be in is the solid S8 ring. Sulfur exists in the gaseous form in five different forms (S, S2, S4, S6, and S8). In order for sulfur to get to these states one must apply a sufficient amount of heat. Two very common oxides of sulfur are sulfur dioxide (SO2) and sulfur trioxide (SO3). These two compounds are used in the production of sulfuric acid. Sulfur also exhibits a wide range of oxidation states, with values ranging from -2 to +6. It is often the central ion in a compound and can easily hold up to 6 atoms around itself. When in the presence of hydrogen it forms the compound H2S which is a poisonous gas, without hydrogen bonds and a very small dipole moment. This reaction with hydrogen epitomizes how different oxygen and sulfur act despite their common valence electron configuration and common nonmetallic properties.
P-block Elements- VI A elements Sulfur Common Compounds of Sulfur with Various Oxidation States
P-block Elements- VI A elements Contrasts in the Chemistry of Oxygen and Sulfur Oxygen is limited to a maximum of four covalent bonds, whereas sulfur forms up to six covalent bonds. For example, oxygen forms only one normal oxide with fluorine, OF2, whereas sulfur forms several compounds with fluorine, including SF6. the oxygen-oxygen double bond is much stronger than the oxygen-oxygen single bond. For sulfur and the other members of the group the energy of two single bonds is greater than that of one double bond. For this reason, multiple bonding is only common for oxygen. TABBond energy Bond energy Oxygen bonds (kJ/mol) Sulfur bonds (kJ/mol) O O 142 S S 268 O=O 494 S=S 425
Contrasts in the Chemistry of Oxygen and Sulfur Oxygen prefer to bond to other elements rather than to itself. For example, the covalent bond to hydrogen is very strong Due to the weakness of the oxygen-oxygen single bond compared to its bonds to other elements Conversely, the sulfur tends to stabilize its compounds by catenation. the sulfur-sulfur single-bond energy is only slightly lower than the energy of sulfur s bonds to other elements Bond energy Bond energy Oxygen bonds (kJ/mol) Sulfur bonds (kJ/mol) O O 142 S S 268 O Cl 218 S Cl 271 O H 459 S H 363
P-block Elements- VI A elements Highlights of Sulfur Chemistry Ozone (O3): ozone, is a very good oxidizing agent. It can be used as a substitute for chlorine in purifying drinking water without giving the water an odd taste. However, because of its unstable nature it disappears and leaves the water unprotected from bacteria. Ozone at very high altitudes in the atmosphere is responsible for protecting the Earth's surface from ultraviolet radiation; however, at lower altitudes it becomes a major component of smog.
P-block Elements- VI A elements Highlights of Sulfur Chemistry Ozone (O3): Preparation of Ozone : It is prepared by passing silent electric discharge through pure and dry oxygen 10 15 % oxygen is coverted to ozone. 3O2(g) 2O3(g); H= +142 kJ/mol Structure of Ozone: Ozone has angular structure. Both O = O bonds are of equal bond length due to resonance.
P-block Elements- VI A elements (a) Two resonance structures for SO2 (b) The SO2 molecule is a bent molecule Highlights of Sulfur Chemistry Sulfur forms two important oxides: SO2 has S in its +4 oxidation state. It is a colorless, choking gas that forms when S, H2S or a metal sulfide burns in air. SO3 has S in the +6 oxidation state. (a) The resonance structures most commonly given for SO3 (b) a resonance structure with three double bonds (c) SO3 is a planar molecule with three equal bonds
P-block Elements- VI A elements Highlights of Sulfur Chemistry SO2 (Sulfur dioxide) Sulfur burns in air to form SO2 Properties: Uses: Refrigerant, preserve dried fruit, bleach for textiles and flour, Gas, Colorless and Poisonous producing sulfuric acid Where the SO2 in our air comes from ~7 1010 kg decomposition of vegetation and volcanic emissions ~1 1011 kg naturally occurring H2S which is oxidized to SO2 by O ~1.5 1011 kg industry and transportation (Electricity Plants) Sulfur can act as either oxidizing agents or an reducing agent. SO2 is the starting material for making sulfur trioxide The SO3 is then used to make sulfuric acid 2SO2(g) + O2(g) 2SO3(g)
P-block Elements- VI A elements Highlights of Sulfur Chemistry Sulfur forms two important oxoacids. Sulfurous acid (H2SO3) is a weak acid with two acidic protons. Sulfuric acid (H2SO4) is a strong acid, it is an important industrial chemical. It is an excellent dehydrating agent. H2SO4 Two resonance structures for H2SO3
P-block Elements- VI A elements Highlights of Sulfur Chemistry H2SO4 (Sulfuric acid) H2SO4 is the most heavily produced inorganic chemical worldwide. About 2/3 of the sulfuric acid produced goes into manufacturing Uses: It is widely used in industry for the production of fertilizers, petrochemicals, and detergents. Three important chemical properties of H2SO4 are that it is; Properties: a strong Bronstead acid (proton donor), Colorless a dehydrating agent, and Corrosive Oily liquid an oxidizing agent.
P-block Elements- VI A elements Highlights of Sulfur Chemistry Sulfur Halides Properties: Sulfur reacts directly with all the halogens except iodine. Colorless SF6 (Sulfur hexafloride) Sulfur reacts spontaneously in fluorine and burns brightly to give SF6 Despite its high oxidation number (+6), It is not a good oxidizing agent Thermally stable Nontoxic gas It is a good insulator in air and is used in switches on high-voltage power lines) SF6 S2Cl2 SF4 S2F10
P-block Elements- VI A elements Group 6A(16) Elements Oxygen, like nitrogen, occurs as a low-boiling diatomic gas, O2. Sulfur, like phosphorus, occurs as a polyatomic molecular solid. Selenium, like arsenic, commonly occurs as a gray metalloid. Tellurium, like antimony, displays network covalent bonding. Polonium, like bismuth, has a metallic crystal structure.