Column Chromatography Methods
Column Chromatography (CC) involves separating solutes based on different mechanisms within a packed column with a mobile phase. The five major types of CC are adsorption, partition, ion exchange, molecular exclusion, and affinity chromatography. Each type utilizes specific stationary and mobile phases to achieve separation based on unique principles. Common terms and techniques associated with column chromatography are also discussed in this overview.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Column Chromatography (CC) This includes chromatographic methods in which: The stationary phase is packed into a column. The mobile phase is a moving liquid or gas. According to the mechanism of separation of solutes, five major types of CC are distinguished. Usually, one mechanism predominates but does not exclude the others
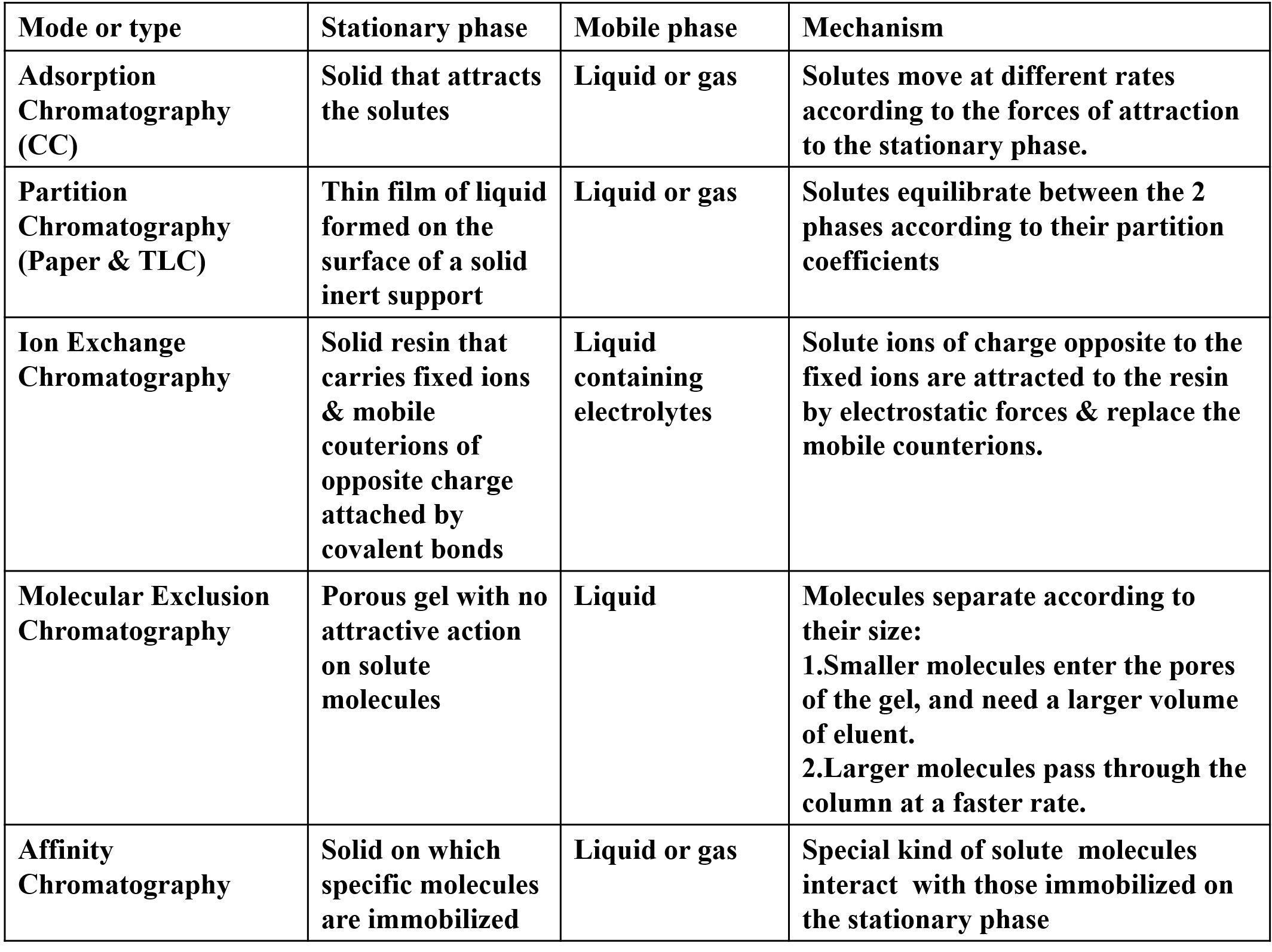
Different Types of chromatography Mode or type Stationary phase Mobile phase Mechanism Adsorption Chromatography (CC) Partition Chromatography (Paper & TLC) Solid that attracts the solutes Liquid or gas Solutes move at different rates according to the forces of attraction to the stationary phase. Solutes equilibrate between the 2 phases according to their partition coefficients Thin film of liquid formed on the surface of a solid inert support Solid resin that carries fixed ions & mobile couterions of opposite charge attached by covalent bonds Porous gel with no attractive action on solute molecules Liquid or gas Ion Exchange Chromatography Liquid containing electrolytes Solute ions of charge opposite to the fixed ions are attracted to the resin by electrostatic forces & replace the mobile counterions. Molecular Exclusion Chromatography Liquid Molecules separate according to their size: 1.Smaller molecules enter the pores of the gel, and need a larger volume of eluent. 2.Larger molecules pass through the column at a faster rate. Special kind of solute molecules interact with those immobilized on the stationary phase Affinity Chromatography Solid on which specific molecules are immobilized Liquid or gas
Column Chromatography Column chromatography Stationary phase is held in a narrow tube through which the mobile phase is forced under pressure or under the effect of gravity
Term Definition Mobile liquid phase with no affinity to the stationary phase (i.e. inert towards it) & no effect on solutes. Solvent Any liquid with more affinity to the stationary phase than the solvent but less than solutes and just capable to move them through the column. Developer Effluent Any liquid that passes out of the column. Any liquid that has lesser affinity to the stationary phase than solutes but is capable to move them out of the column. Eluent Eluate Fraction of eluent containing a required specific substance. (or retardation volume): Volume of mobile phase that passes out of the column, before elution of a specific substance. Retention volume (VR)
Open Column Chromatography (Traditional column chromatography) Traditional column chromatography is characterized by addition of mobile phase under atmospheric pressure and the stationary phase is packed in a glass column.
Packing & operating the column 1- Packing The selection of the method of packing depends mainly on thedensity of the solid.Techniques used are the wet, dry & slurry methods. In all cases avoid inclusion of air bubbles
2- Sample Application Apply evenly & in a concentrated solution to the top of the column which is protected from disturbance (e.g. add glass wool or filter paper).
Elution techniques .1 Technique Procedure Isocratic elution Addition of solvent mixture of fixed composition during the whole process. Gradient elution Continuous or linear elution: in which there is continuous change in the composition of the mobile phase over a period of time (e.g. polarity, pH or ionic strength). Step wise or fractional elution: in which the change is not continuous i.e. a sudden change in the composition of the mobile phase is followed by a period where the mobile phase is held constant.
4- Detection On-column detection for colored or fluorescent compounds directly after developing the chromatogram. Monitoring of eluted fractions (PC or TLC). Using special detectors connected to the column such as refractive index, UV detectors, etc
Factors affecting solutes separation in CC ( Factors affecting column efficiency) Factor Effect Particle size of solid stationary phase (or of support) Column dimensions Decrease of size improves separation (but very small particles need high pressure). Efficiency increases as ratio length / width increases. Non uniform packing results in irregular movement of solutes through column & less uniform zone formation, (i.e. band broadning or tailing). Increase in column temperature results in speed of elution but does not improve separation (tailing). Solvents should be of low viscosity (to give efficient resolution) & high volatility (to get rapid recovery of the substances). Uniform & low flow rate gives better resolution. Discontinuous flow disturbs resolution Deactivation of adsorbent decreases separation. Substances of high concentration move slowly. Uniformity of packing Column temperature Eluting solvent Solvent flow rate Continuity of flow Condition of adsorbent Concentration of solutes
Adsorption Column Chromatography Adsorbents: The most common are Alumina & Silica gel in which the interactions with solute molecules is due to OH groups presenton their surface. More polar molecules are adsorbed morestrongly & thus, will elute more slowly Strength of adsorption of polar groups (solutes) on polar support is in the following order: -C=C- < O-CH3 < -COOR < >C = O < -CHO < -NH2 < -OH < -COOH Olefins < Ethers < Esters < Lactones < Aldehydes < Amines < Phenols < Acids.