Essentiality of Cr(VI)-Induced Mutagenesis in Small Intestinal Tumor Development in Mice
Cr(VI)-induced mutagenesis is a key event in the mode of action for small intestinal tumors in mice, with the question of whether it is an essential step in tumor development. The interaction of cellular components with Cr leads to cell proliferation, hyperplasia, and ultimately mutagenesis. Different hypotheses suggest mutations occurring directly due to Cr-induced DNA damage or as a result of failure in DNA repair mechanisms. Understanding the essentiality of mutagenesis in tumor induction requires evaluating experimental evidence in affected tissues and considering the downstream key events and their prevention if upstream events are blocked. The EPA MOA Framework emphasizes assessing tissue-specific data to determine the role of mutagenesis in tumor formation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
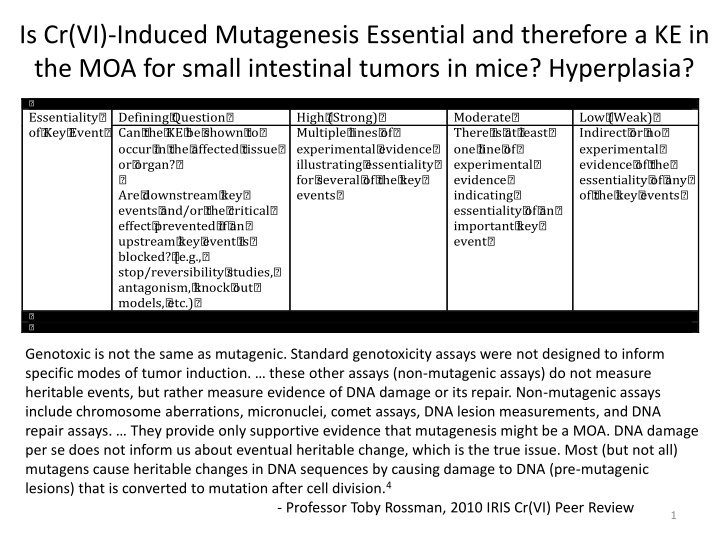
Is Cr(VI)-Induced Mutagenesis Essential and therefore a KE in the MOA for small intestinal tumors in mice? Hyperplasia? ? Essentiality? of? Key? Event? Defining? Question? Can? the? KE? be? shown? to? occur? in? the? affected? tissue? or? organ?? ? Are? downstream? key? events? and/or? the? critical? effect? prevented? if? an? upstream? key? event? is? blocked?? [e.g.,? stop/reversibility? studies,? antagonism,? knock? out? models,? etc.)? High? (Strong)? Multiple? lines? of? experimental? evidence? illustrating? essentiality? for? several? of? the? key? events? Moderate? There? is? at? least? one? line? of? experimental? evidence? indicating? essentiality? of? an? important? key? event? Low? (Weak)? Indirect? or? no? experimental? evidence? of? the? essentiality? of? any? of? the? key? events? ? ? Genotoxic is not the same as mutagenic. Standard genotoxicity assays were not designed to inform specific modes of tumor induction. these other assays (non-mutagenic assays) do not measure heritable events, but rather measure evidence of DNA damage or its repair. Non-mutagenic assays include chromosome aberrations, micronuclei, comet assays, DNA lesion measurements, and DNA repair assays. They provide only supportive evidence that mutagenesis might be a MOA. DNA damage per se does not inform us about eventual heritable change, which is the true issue. Most (but not all) mutagens cause heritable changes in DNA sequences by causing damage to DNA (pre-mutagenic lesions) that is converted to mutation after cell division.4 - Professor Toby Rossman, 2010 IRIS Cr(VI) Peer Review 1
Two Competing MOA Hypotheses McCarroll et al. (2010)2 Interaction of Cellular Components with Cr Cell Proliferation / Hyperplasia Gastrointestinal Tumors Mutagenesis Mutations occurring as a direct result of Cr-induced DNA damage Thompson et al. (2013)3 MOA for Cr(VI) in mouse small intestines 253 DOI: 10.3109/10408444.2013.768596 Mutations fixed due to failure of DNA repair from shortened cell cycle 2 Figure 2. Proposed MOA for Cr(VI) carcinogenesis in the small intestine. that is itself a necessary element of the mode of action or is a biologically based marker for such an element . The EPA MOA Framework, like those proposed by the other scientists and organizations (Boobis et al., 2006, 2008; Meek & Klaunig, 2010; Sonich-Mullin et al., 2001), stresses that the MOA for each tumor site should be evaluated separately. Moreover, toxicology data should ideally be evaluated (or generated if absent) in the target tissues of interest. Therefore, the MOA described below focuses on data obtained from the duodenal and jejunal intestinal mucosae of mice. 1987; Proctor et al. 2012). Cr(VI) that escapesreductioninthe stomachwill betransportedtothesmall intestineandtakenup into intestinal enterocytes, or transited along thelumen of the gastrointestinal tract where it can be reduced by microbiota (Shrivastava et al., 2003) and intestinal secretions such as cysteine (Dahm & Jones, 2000), and excreted in feces. Following Cr(VI) administration in drinking water, pharma- cokinetic data indicate low chromium tissue burdens in the ileum, higher levelsinthejejunumandstill higher levelsinthe duodenum (Figure 3A), likely demonstrating that Cr(VI) absorption decreases distally through the small intestine due to(1)reductionof Cr(VI)toCr(III) intheintestinal lumen,and/ or (2) differencesintherelativeabsorptionof chromium inthe intestinal segments (Kirman et al., 2012; Thompson et al., 2011b). Consistent with these data, tissue damage and tumor formation areincreased mostly intheduodenum, only slightly inthejejunumandnot increasedintheileumor largeintestine (NTP, 2007, 2008b). In vivo and ex vivo pharmacokinetic data collected in the alimentary tract of mice provide evidence that the carcino- genic dosesintheNTPstudy depletethereductivecapacity of the mouse gastric contents (Proctor et al., 2012; Thompson et al., 2011b). Cr(VI) cannot bespeciated intissues; therefore, only the total chromium concentration in tissues can be measured. However, because Cr(VI) is much better absorbed than Cr(III), a significant increase in tissue total chromium concentrations suggests that extracellular reducing capacity was exceeded and that higher concentrations of Cr(VI) are available for absorption. After 90 daysof exposure to Cr(VI) in drinking water, duodenal chromium levels were not significantly increased at the two lowest treatment doses (Figure 3A). At these doses, the physiologically based pharmacokinetic (PBPK) model developed for chromium in mice (Kirman et al., 2012) predicts that the amount of reducing equivalents (lumped concentration of reducing agents) in the gastrointestinal lumen of mice is relatively unchanged compared to controls (Figure 3B). In contrast, exposure to the three highest doses of Cr(VI) in drinking water, which were carcinogenic in the NTP 2-year bioassay (NTP, 2008b), resulted in a significant increase in duodenal total Cr tissue concentrations from 5 0.02m g/g (controls) to 34, 42 and 61m g/g, respectively (Figure 3A and B). At the three highest doses administered to mice, the PBPK model (Kirmanet al., 2012) predictsthat Cr(VI) exposureproducesa substantial depletion inthereducingequivalentspresent inthe gastrointestinal lumen (Figure 3B). Critical Reviews in Toxicology Downloaded from informahealthcare.com by 72.152.41.33 on 04/20/14 Summary of the key events in the MOA for intestinal tumors For personal use only. The histological, biochemical, toxicogenomic and pharmaco- kinetic data collected as part of our MOA studies were evaluated along with other relevant data in the published literature using the MOA and human relevance frameworks developed by the US EPA and other organizations. Based on thisassessment, weconcludethat theoverall WOE supportsa cytotoxic nonmutagenic MOA with the key events shown in Figure 2: Key event 1: Absorption of Cr(VI) from the intestinal lumen; Key event 2: Villouscytotoxicity; Key event 3: Sustained compensatory crypt hyperplasiato repair/replace thedamagedintestinal mucosa; and Key event 4: Mutagenesis within crypt cells, ultimately leading to tumorigenesis. Each of these key events, and supporting data, are summarized in the following sections. Key event 1: Absorption from the lumen In most physiological conditions, Cr(VI) exists primarily as chromateanions(CrO2 (SO2 through anion transporters (De Flora, 2000; Markovich, 2001; Salnikow & Zhitkovich, 2008; Zhitkovich, 2005). Cr(III) isnot structurally similar to sulfateand phosphateand thus enters cells more passively. Extracellular reduction of Cr(VI) to Cr(III) prevents cellular absorption through these transportersandisawell-recognizedkineticprocessthat limits toxicity (De Flora, 2000; De Flora et al., 1997; Donaldson & Barreras,1966; Febel etal.,2001; Kergeret al.,1996; USEPA, 1998). Thestoichiometry of Cr(VI) reduction to Cr(III) in the lumenof thestomachandsmall intestineisnotfullyknown,but reduction capacity varies depending on stomach conditions includingpHandwhether inafedorfastedstate(DeFloraetal. 4) that arestructurally similar tosulfate 4) and phosphate (PO2 4), and therefore, enter cells
Evidence Integration Including evidence of DNA Damage or Mutation Supporting Evidence / Damage Point mutations in bacteria (Ames Test)2 DNA damage in liver, WBCs, brain, skin and bone marrow of mice2 Increases in 8-OHdG and DNA-protein crosslinks (DPXL) in isolated mouse duodenal cells treated for 1 hr in vitro5 Supporting Evidence / Mutation Positive mammalian spot test (coat color change in offspring) in mice exposed to welding fumes containing chromate6 Mutations in bone marrow and liver of transgenic mice exposed to a single concentration of Cr(VI) measured by packaging into a phage, infection of E. coli and occurrence of mutant plaques7 Refuting Evidence / Damage Lack of any dose-related change in 8-OHdG adducts in duodenal epithelium of mice treated for 3 months8 No change in 8-OHdG adducts or DPXL in mouse duodenal epithelium after 9 months of drinking water exposure to 5 or 20 mg/L Cr(V)) for 9 months5 Refuting Evidence / Mutation Lack of a dose-related effect of K-Ras codon 12 GGT->GAT mutation in mouse duodenal epithelium9 The genomic signature and expression of 4 genes involved in the DNA damage response induced by Cr(VI) in mouse duodenal epithelium were much more similar to those of non-mutagenic carcinogens than to mutagenic carcinogens10 3
Why Including these Papers is Critical? If a mutagenic MOA is assumed, linear low-dose extrapolation along with an age-dependent adjustment factors will result in an overestimate of Cr(VI) carcinogenicity. Assuming a non-mutagenic MOA will likely result in choosing a precursor or sentinel key event and the use of non-linear low-dose extrapolation resulting in an RfD protective of cancer, similar to that for chloroform. (p. 32-33 of NAS Review) The Arsenic preliminary materials provide a starting point for MOA consideration. 4