Enhancing Plain Language Summaries for Consumer Involvement
Improving plain language summaries is crucial for supporting consumer involvement in healthcare decision-making. This presentation highlights the importance of clear communication, showcasing the impact of enhanced summaries and addressing challenges in achieving consistency and quality. Key areas of focus include sentence length, reading levels, complex terms, and feedback mechanisms to refine standards. The goal is to empower consumers with accessible information for informed choices.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Catherine McIlwain (cmcilwain@cochrane.org) 20thAnnual Cochrane Colloquium Auckland, 3rdOctober 2012
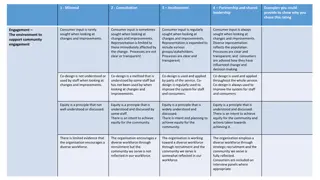
Improving plain language summaries Supporting consumer involvement Getting the word out
Random sample of 243 plain language summaries (PLS) 24% abstracts didn t match 24% of the plain language summaries and *German Cochrane Centre survey 2004
Use of Statistics Explaining Bias Quality of Evidence Imprecision
*Excerpt from Glenton and Santesso presentation to the Co- ordinating Editors Board in 2010 October.
Sentence length Reading level Headers and paragraphs Use of complex terms
Consumer Feedback Essential Consumer Feedback Essential Consumer developed ERC refined Adherence to MECIR and PLS standards Pilot phase in progress
Including an explanatory guide to accompany the Consumer Referee Checklist
s summarie ummaries.cochrane.org s.cochrane.org
European Commission ECRAN Project Communicating the benefits of European research to the general public
E European uropean C Communication on ommunication on R Research A Awareness wareness N Needs eeds Communicating the benefits of European research to the general public esearch To develop tools to: communicate key messages to citizens, patients, patients organizations, healthcare professionals, researchers, policymakers and society about independent, multinational clinical research First key message: The importance of public understanding of the need for, and basic principles of clinical trials, fostering active involvement of citizens and patients in trials and of their representatives in trial design