Deconstructing Stereochemistry in Chemical Reaction
Learn step-by-step methods to deconstruct the stereochemistry of a proposed reaction. Convert Fischer structure to 3D projection, analyze attack by reagent, achieve inversion, and rotate Fischer projection.
Uploaded on Feb 23, 2025 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
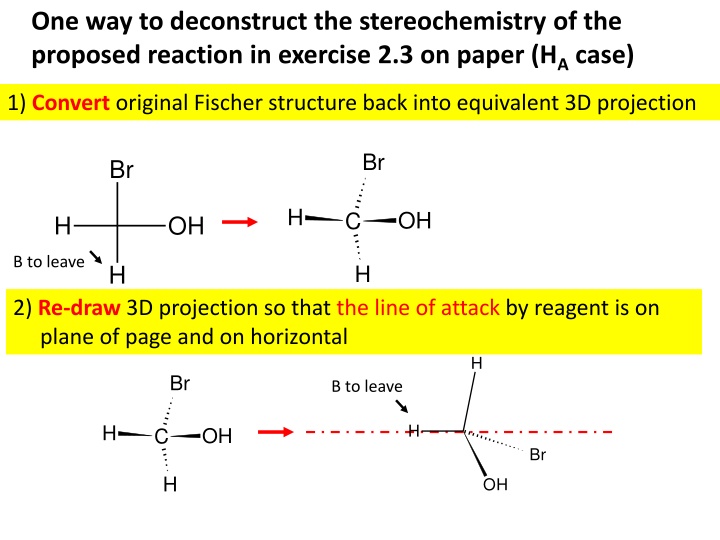
One way to deconstruct the stereochemistry of the proposed reaction in exercise 2.3 on paper (HA case) 1) Convert original Fischer structure back into equivalent 3D projection Br Br H OH C H OH B to leave H H 2) Re-draw 3D projection so that the line of attack by reagent is on plane of page and on horizontal H Br B to leave H H OH C Br H OH
One way to deconstruct the stereochemistry of the proposed reaction in exercise 2.3 (cont.) 3a) Bring in attacking Cl* along horizontal and deform the molecule consistent with the expected inversion H H Cl Cl H H Br Br H O OH 5-coordinate- activated complex 3b) Finish reaction by completing inversion and ejecting HA . H Cl Br H O Inverted product (HA gone)
One way to deconstruct the stereochemistry of the proposed reaction in exercise 2.3 (cont.) 4) Rotate projection so that Fischer orientation is maintained, and, so that smallest group is on top along the vertical. Here, we must swivel the molecule on its `Y axis so that the OH and Cl and OH come out of the plane and H and the Br turn into the plane H H y Cl x Cl Br H O H O Br z 5) Re-draw Fischer projection and assign H 16 H O Cl 35.4 S Br 80