Chemistry Fundamentals: Elements, Mixtures, Metals, and Changes
Explore fundamental concepts in chemistry including elements found on the periodic table, types of mixtures, characteristics of metals and non-metals, periodic table groups, chemical vs. physical properties, and changes in matter. Learn about the properties and behaviors of elements, compounds, and mixtures, as well as how to distinguish between chemical and physical changes in substances.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chemistry Review Alexander Crumpton Fuqua
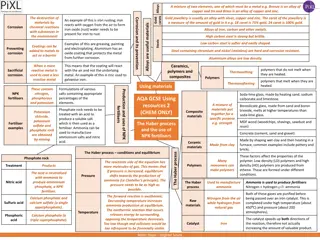
Elements Pure substances Found on the periodic table Na, Li, Be, B, Fe, Au, etc Compounds Mixtures Pure substance 2 or more substances Chemically bound Physically combined Separate through chemical reactions Easily separated Filter, evaporate, sift 2 or more elements H20, NaCl, CO2, Fe2O3
Types of Mixtures Homogeneous Mixture Heterogeneous Mixture Can identify all the parts mixed in Also called a solution Can not see the different parts Salad, Trail mix, granite, sand, Italian dressing, chicken soup Evenly mixed Air, steel, bronze, saltwater
Metals Reactive Left of the staircase Shiny/luster Good conductors of heat and electricity Malleable Ductile Magnetic Non-Metals Metalloids Dull Sit on the staircase Brittle Semi-conductors Right of the stair case Share characteristics Poor Conductors Reactive with others
Periodic Table Bonding Families USUALLY Group 1 Group 17 Group 2 Group 16 Group 13 Group 15 Group 14 Group 14 Arranged according to increasing atomic number Group 1: Alkali Metals Most reactive metals Group 2: Alkaline Earth Metals Groups/Families Share Characteristics Reactive metals, but less than group 1 Group 3-12: Transition Elements 1-92 Natural Elements Most varied properties 93 or higher- Synthetic Elements Most familiar elements Group 17: Halogens Most reactive NON-METAL Group 18: Noble Gases/Inert Gases Least/Non-reactive group of elements Found in elemental form in nature
Chemical vs. Physical Properties Chemical Properties Physical Properties Can only observe by changing the object to something else, a new kind of matter Can be observed WITHOUT making it something NEW Can observe with the 5 Senses Shape Density Solubility Odor Melting Point Boiling Point Color Reacting with oxygen Burning Rusting Apple turning brown Reacting with Acids
Chemical vs. Physical Change Chemical Change Physical Change New substance is created Change in size, shape or form Properties different from original No new substance is created How you know a change has occurred: Color change Temperature change Formation of a precipitate Formation of a gas
Endothermic vs. Exothermic Reactions Endothermic Reactions: Exothermic Reactions: Takes in energy (heat) Examples: Melting Evaporating Cooking Gives off energy (heat) to the surroundings Examples: Combustion Composting Candle Flame
Law of Conservation of Matter (Mass) Matter is not created or destroyed in ordinary chemical and physical changes Open System Closed System Matter is lost to the surrounding environment i.e. mixing baking soda and peroxide in an open beaker, allowing gas to escape Matter is not lost to the surrounding environments i.e. catching the smoke and ashes to measure out the final weight of the products
Balancing Equations Write out Elements in equations Count # of atoms present Start at the left, change coefficients (NEVER SUBSCRIPTS) in order to balance Continue balancing from left to right 2 H2O 2 H2O + 1 O2
Some Chemical reactions occur in more than one step. A 2 step reaction is shown Step 1: 2 PO2 PO3 + PO 30 grams x grams 7.62 grams Assuming all PO3 produced in step 1 completely reacts in step 2, approximately how many grams of CO2 are produced in step 2? Step 2: CO PO3 + PO2 + CO x grams 5.09 grams 10 grams y grams
Some Chemical reactions occur in more than one step. A 2 step reaction is shown Step 1: 2 PO2 PO3 + PO 10 grams x grams 4.83 grams Assuming all PO3 produced in step 1 completely reacts in step 2, approximately how many grams of CO2 are produced in step 2? Step 2: CO PO3 + PO2 + CO x grams 7.29 grams 5 grams y grams