Atomic Electron Shells and Energy Levels
Exploring how electrons move between energy levels in atoms, providing insights into electron shells and emission spectra. The progression from heat energy to light emission is visualized, shedding light on the arrangement of electron shells and the concept of energy level diagrams.
Uploaded on Sep 25, 2024 | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
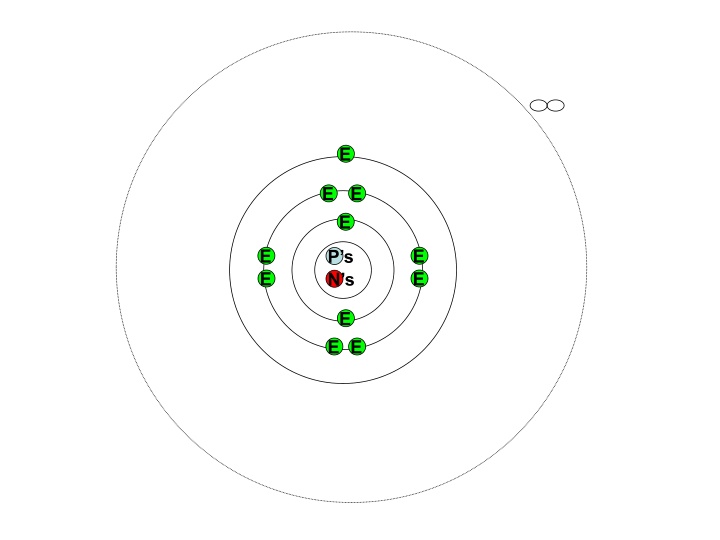
E E E E E E P s N s E E E E E
Electron promoted from heat energy from the Bunsen burner
Energy given out as light as the electron falls back to its original position
We think of an atom like this. Having electron shells at equal distances apart If it was like this then surely the lines in the spectra would be equally spread out.
The line emission spectra tells us something different. The red is of lower energy so would represent the electron shell nearest the nucleus Rotate the spectra Each new line represents a new electron shell. As you move away from the nucleus they get closer together.
So the electron shells must look more like this
We can change the diagram to make it easier to see what is going on in the atom We only need to look at one section of the atom:
We can change the diagram to make it easier to see what is going on in the atom We only need to look at one section of the atom: From here, we extend the curves into straight lines. This gives us a chart called an energy level diagram:
5th electron shell 4th electron shell 3rd electron shell 2nd electron shell 1st electron shell
5th electron shell 4th electron shell 3rd electron shell 2nd electron shell 1st electron shell
Energy 5th electron shell etc 4th electron shell n=4 n=3 3rd electron shell We call these diagrams energy level diagrams and each electron shell is now called The Principle Quantum Number, n 2nd electron shell n=2 1st electron shell n=1
Energy 5th electron shell 4th electron shell 32e 18e 3rd electron shell At GCSE you only filled the first 2 electron shells: 2.8. But if you keep filling they can hold more electrons (because they are bigger). We will look at this in more detail later. 2nd electron shell 8e 1st electron shell 2e To work out the number of electrons = 2n2