Acids and Bases in Chemistry
Learn about the properties of acids and bases, how indicators are used to identify them, and conduct experiments to differentiate between acids, bases, and neutral substances. Explore the relationship between acid indigestion and antacids, and understand the corrosive nature of acids and bases. Discover how to classify substances based on observable behavior and color changes with indicators in this engaging chemistry unit focused on toxins and solution chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 4: TOXINS Stoichiometry, Solution Chemistry, and Acids and Bases
Lesson 84: Heartburn Acids and Bases
ChemCatalyst Countless products are advertised on TV with the promise of reducing acid indigestion. a. What is acid indigestion? b. What does acid have to do with your stomach? c. How do you think antacids work?
Key Question What are the properties of acids and bases?
You will be able to: identify acids and bases based on general observable properties explain how an indicator is used to determine whether a solution is acidic, basic, or neutral
Prepare for the Lab Work in groups of four. Indicator: An indicator is a molecular substance that changes color when it comes into contact with an acid or a base. Acids and bases are corrosive. Do not get solutions on skin. In case of a spill, rinse thoroughly with water. Wear safety goggles at all times.
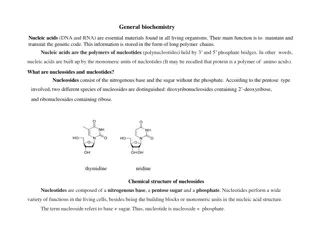
Discussion Notes The substances on the left side of the number line (from 0 to 7) are all acids. The substances on the right side of the number line (from 7 to 14) are called bases. The substances located in the middle of the number line (around 7) are called neutral substances.
Wrap Up What are the properties of acids and bases? Acids and bases are solutions that are classified according to their observable behavior. Acids and bases change the color of indicators. Substances that are not acids or bases are considered neutral.
Check-In An unknown substance is mixed with cabbage juice, and the solution turns purple. The substance does not react with calcium carbonate. Is it an acid, a base, or a neutral substance? Explain.