Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or ketones. Chain-walking via palladium catalysis and other aspects of the Heck reaction are also discussed in this pedagogical and informative content from the University of Utah's Sigman Lab.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
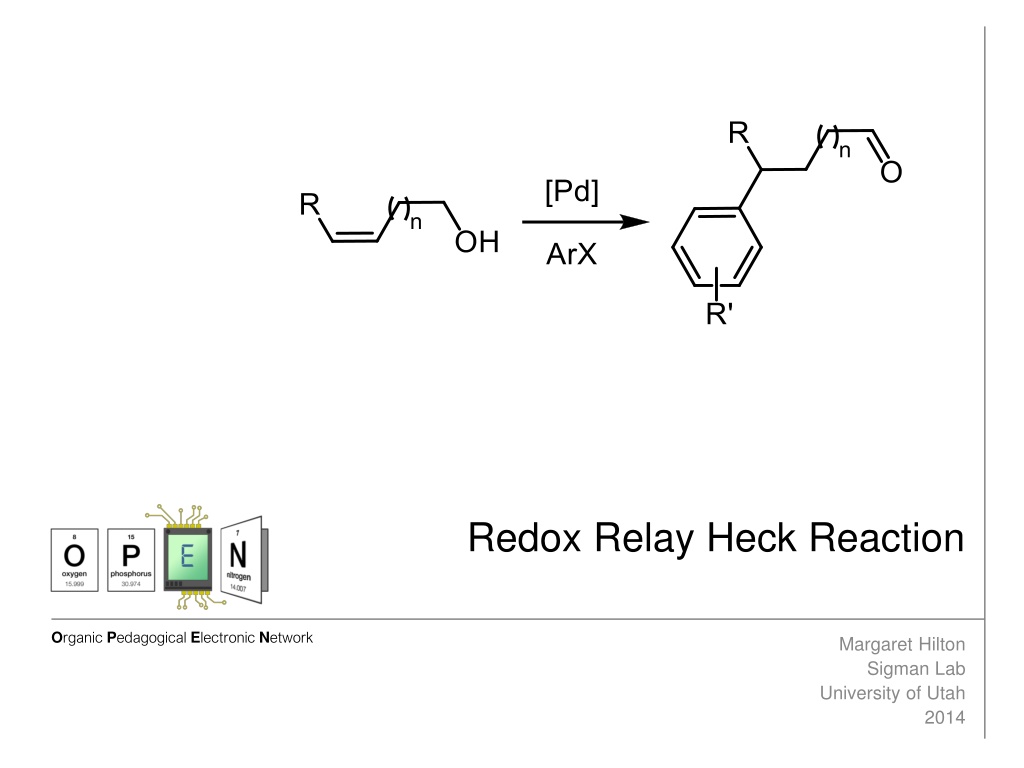
Redox Relay Heck Reaction Organic Pedagogical Electronic Network Margaret Hilton Sigman Lab University of Utah 2014
Heck Reaction Since Heck s seminal publication in 1968, the Heck reaction has been a powerful synthetic tool for the functionalization of olefins with aryl groups. General Mechanism* Heck s Conditions: Heck, along with Negishi and Suzuki, won the Chemistry Nobel Prize in 2010 for their work in cross coupling. Review: Chem. Rev. 2000, 100, 3009. Other Examples and Applications: Overman JACS 1993, 115, 11028 , Sarpong JACS 2008, 130, 7222, Shibasaki JOC 1996, 61, 4876, Sigman JACS 2010, 132, 13981, Sigman JACS 2011, 133, 9692. 2010 Nobel Prize *Note: this reaction may also proceed through an oxidative mechanism, where a PdII precatalyst , a transmetallating aryl reagent, and a terminal oxidant are used, such as with Heck s conditions shown.
Chain-Walking Examples of Relay via Palladium Catalysis After the initial migratory insertion, which forms a C-C bond, a -hydride elimination will occur, producing a Pd-H intermediate. If the alkene remains coordinated to palladium and the hydride reinserts at the opposite carbon, the palladium catalyst is now positioned one more carbon down the alkyl chain. This is called a relay or chain walking and produces alkene isomers. Can chain-walking be controlled? Hayashi JACS 1991, 113, 1417 Curran, JACS 2007, 129, 494
Redox-Relay Heck Reaction Sigman and coworkers have developed a redox-relay Heck reaction, where the relay by palladium is controlled by a thermodynamic sink (an alcohol) on the substrate. The unsaturation of the alkene is transferred to the alcohol to form aldehydes or ketones. Classical Variant Oxidative Variant Sigman Science2012, 338, 1455 Sigman JACS, 2013, 135, 6830
Problems 1. Keay, JOC, 2007, 72, 7253
Contributed by: Margaret Hilton Sigman Lab University of Utah 2014 This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License.