Trilogy Plenary Extended Writing Questions in Biology, Chemistry, and Physics
Explore detailed questions and content covering Biology topics such as Cell Biology, Transport in Cells, Animal Tissues, Organs and Organ Systems, Infection & Response, Bioenergetics, Homeostasis, and Ecology. Dive into Chemistry and Physics sections for a comprehensive study on various topics. Understand key concepts with clear explanations and logical reasoning, enhancing your knowledge in the sciences.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Trilogy Plenary Extended Writing Questions
Sections Biology Chemistry Physics
Biology Paper 1 4.1 Cell Biology Transport in cells 4.2 Organisation Animal tissues, organs and organ systems 4.3 Infection & Response Communicable Diseases 4.4 Bioenergetics Photosynthesis Respiration Paper 2 4.5 Homeostasis Nervous System Hormonal Control 4.6 Inheritance, variation and evolution Reproduction Variation & Evolution 4.7 Ecology Adaptation Organisation of an ecosystem Biodiversity
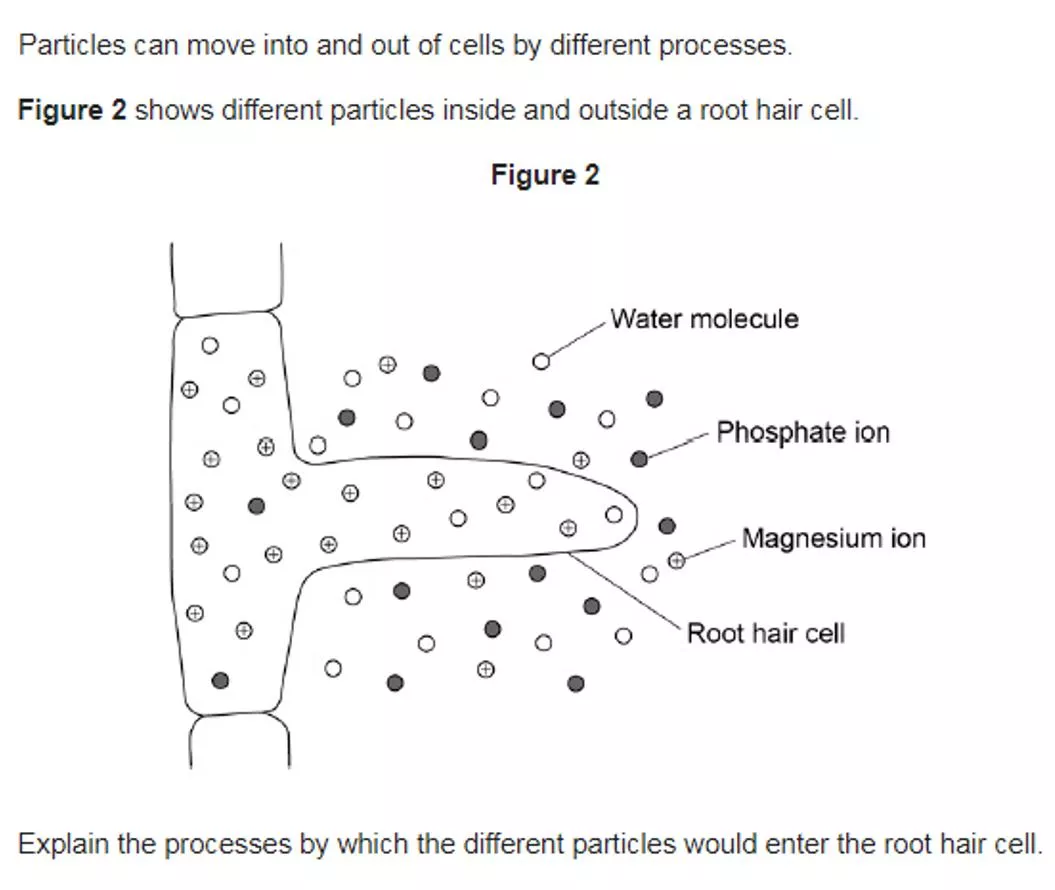
4.1 Cell Biology Transport in cells Level 3: Relevant points (correct processes / explanations) are identified, given in detail and linked logically to form a clear account. 5-6 Level 2: Relevant points (correct processes / explanations) are identified and there are attempts at logical thinking. The resulting account is not fully clear. 3-4 Level 1: Points are identified and stated simply, but their relevance is not clear and there is no attempt at logical thinking. No relevant content 1-2 0 Indicative content water is absorbed by osmosis osmosis is a passive process, or described water in soil is at a higher concentration than inside cell water moves down concentration gradient through a partially permeable membrane phosphate ions absorbed by diffusion diffusion is a passive process, or described phosphate ions are in a higher concentration in soil than inside cells magnesium ions are absorbed by active transport magnesium ions are in lower concentration in soil than inside cells magnesium ions move from an area of lower concentration to an area of higher concentration inside the cells magnesium ions move up the concentration gradient process requires energy energy from respiration
4.2 Organisation Animals, tissues, organs and organ systems Arteries and veins have different structures and different functions. Explain how the different structure of arteries and veins relates to their different functions.
4.2 Organisation Animals, tissues, organs and organ systems Level 3: Relevant points (differences / functions) are identified, given in detail and linked logically to form a clear account. 5-6 Level 2: Relevant points (differences / functions) are identified and there are attempts at logical linking. The resulting account is not fully clear. 3-4 Level 1: Points are identified and stated simply, but their relevance is not clear and there is no attempt at logical linking. No relevant content Indicative content artery has a thicker wall (because) artery has to withstand higher pressure artery has thicker layer of elastic tissue / fibres (so) it can stretch (so) artery returns to original size / shape artery has thicker layer of muscle to maintain a force on the blood vein has valves (valves) prevent backflow of blood artery carries blood away from the heart vein carries blood towards the heart ignore references to oxygenated / deoxygenated blood 1-2 0
4.3 Infection & Response Communicable Diseases An antibiotic needs further testing before it can be licensed for human use. The first stage is pre-clinical tests using live cells, tissues and animals. Describe the other stages of drug testing. Give reasons for each stage.
4.3 Infection & Response Communicable Diseases Level 3: Relevant points (correct stages / reasons) are identified, given in detail and linked logically to form a clear account. 5-6 Level 2: Relevant points (correct stages / reasons) are identified and there are attempts at logical thinking. The resulting account is not fully clear. 3-4 Level 1: Points are identified and stated simply, but their relevance is not clear and there is no attempt at logical thinking. 1-2 No relevant content Indicative content names of stages are not required, but a logical progression through stages of testing is required for Levels 2 and 3. phase 1 clinical testing: tested on healthy volunteers low doses used reason: to test for side effects / toxicity / safety phase 2 clinical testing: tested on patients patients given placebo or drug double blind trial reason: to test for side effects / toxicity / safety to test its efficacy / effectiveness phase 3 clinical testing: larger numbers of patients used patients given placebo or drug double blind trial reason: to verify efficacy / effectiveness to determine correct dose prior to licensing: analysis of results peer review reason: to check results are valid to avoid bias 0
4.4 Bioenergetics - Photosynthesis Figure 1 shows some of the apparatus that can be used to measure the rate of photosynthesis. The rate of photosynthesis in the pondweed is affected by different colours of light. Describe a method you could use to investigate this. You should include: what you would measure variables you would control.
4.4 Bioenergetics - Photosynthesis Level 3 (5 6 marks): A coherent method is described with relevant detail, which demonstrates a broad understanding of the relevant techniques and procedures. The steps in the method are logically ordered. The method would lead to the production of valid results. Level 2 (3 4 marks): The bulk of the method is described with mostly relevant detail, which demonstrates a reasonable understanding of the relevant scientific techniques and procedures. The method may not be in a completely logical order and may be missing some detail. Level 1 (1 2 marks): Simple statements are made which demonstrate some understanding of some of the relevant scientific techniques and procedures. The response may lack a logical structure and would not lead to the production of valid results. 0 marks: No relevant content Indicative content description of how the apparatus would be used reference to control intensity of light / brightness use of ruler to measure distance of light from beaker / pondweed reference to varying colour of light or use of different filters plant releases gas / oxygen measure number of bubbles / volume of gas produced same length of time reference to control of temperature reference to control / supply of carbon dioxide in water do repeats and calculate a mean
4.4 Bioenergetics - Respiration When maggots respire they take in a gas from the air and release a different gas. Solution A absorbs the gas released. At the start of the investigation the student records the distance of the water droplet from the bend in the capillary tube. Explain what happens to the water droplet as the maggots respire.
4.4 Bioenergetics - Respiration Level 2 (3 4 marks): A detailed and coherent explanation is given of how the droplet moves, clearly and logically linked to the process of respiration. Level 1 (1 2 marks): Simple statements are made about movement of the water droplet, but any attempts at explaining the reason or linking the movement to the process of respiration are unclear and poorly structured. 0 marks: No relevant content Indicative content water droplet moves towards the maggots / boiling tube Explanation: the oxygen in the boiling tube is used up in respiration (and) the carbon dioxide released from respiration is absorbed by solution A which causes a pressure difference so air is drawn into the tube bringing the water droplet with it.
4.5 Homeostasis The human nervous system Two students investigated the effect of caffeine concentration on reaction time. This is the method used. 1. Student A drinks a cup of coffee. 2. Student B holds a ruler above Student A s hand. 3. Student B drops the ruler. 4. Student A catches the ruler as quickly as she can. 5. The distance the ruler falls is recorded. Suggest how this method could be improved to produce valid results.
4.5 Homeostasis The human nervous system Level 3 (5 6 marks): A coherent method is described with relevant detail, which demonstrates a broad understanding of the relevant scientific techniques and procedures. The steps in the method are logically ordered. The method would lead to the collection of valid results. Level 2 (3 4 marks): The bulk of a method is described with mostly relevant detail, which demonstrates a reasonable understanding of the relevant techniques and procedures. The method may not be in a completely logical sequence and may be missing some detail. Level 1 (1 2 marks): Discrete relevant points are made which demonstrate some understanding of the relevant scientific techniques and procedures. They may lack a logical structure and would not lead to the production of valid results. 0 marks: No relevant content. Indicative content use decaffeinated coffee as control control volume of coffee blind trial or do not tell students which coffee they are drinking left for standard time between drink and test at least 10 minutes control start position of ruler control other factors such as light in the room same person for different concentrations repeat for each caffeine concentration use a range of caffeine concentrations start with lowest concentration of caffeine use caffeine solution instead of coffee to control for other ingredients repeat investigation with more people and calculate means
4.5 Homeostasis Hormonal coordination in humans In Vitro Fertilisation (IVF) treatment can be used to help women become pregnant. IVF uses some of the hormones shown in the figure above. Explain why IVF increases the chance of some women becoming pregnant.
4.5 Homeostasis Hormonal coordination in humans Level 3 (5 6 marks): A detailed and coherent explanation is given, which logically links the role of different hormones to their use in IVF and a clear explanation of how IVF increases the chance of a successful pregnancy. Level 2 (3 4 marks): An attempt is made to link the role of hormones to their use in IVF. The logic used in explaining how IVF increases the chance of a successful pregnancy may not be clear or linked to the hormones. Level 1 (1 2 marks): Discrete relevant points made. The logic may be unclear and links may not be made. 0 marks: No relevant content Indicative content Identification of hormones used in IVF: FSH LH. Role of hormones in IVF: FSH causes eggs to mature LH causes the eggs to be released. Effect on chance of successful pregnancy: high levels of hormones cause many eggs to be matured and released sperm and eggs are collected and eggs are fertilised (so increased probability of fertilisation) fertilised eggs are given time to develop into a small ball of cells some are transferred into the mother (uterus), to increase the probability of one successfully implanting.
4.6 Inheritance, variation and evolution - Reproduction A couple who could pass on Huntington s disease visit a genetic counsellor, who gives them the following options. 1. Adopt a child. 2. Gamete donation uses sperm from another man to fertilise the woman s eggs by in vitro fertilisation (IVF). 3. Conceive naturally. 4. Use pre-implantation genetic diagnosis (PGD). Many embryos are produced by IVF using gametes from the man and woman. Embryos are tested for Huntington s disease and a healthy embryo is implanted into the woman s uterus. The risk of implanting an embryo with the allele for Huntington s disease is 0.2%. Costs the NHS about 11 000. 5. Conceive naturally and use prenatal diagnosis (PND) once the woman becomes pregnant. A sample of the placenta is taken at 10 weeks of pregnancy or a sample of fluid is taken from around the developing baby at 16 weeks of pregnancy. The sample is tested for the Huntington s allele. A 0.5 1.0% risk of miscarriage. About 1% of samples collected are unsuitable for testing. Costs the NHS about 600. The couple decide they want to have a healthy baby that is their own biological offspring. Evaluate the options. Suggest which option would be best for the couple. (c)
4.6 Inheritance, variation and evolution Reproduction part 1 Level 3 (5 6 marks): A detailed and coherent evaluation is provided which considers a range of relevant points and comes to a conclusion consistent with the reasoning. Level 2 (3 4 marks): An attempt is made to relate relevant points and come to a conclusion. The logic may be inconsistent at times but builds towards a coherent argument. Level 1 (1 2 marks): Discrete relevant points made. The logic may be unclear and the conclusion, if present, may not be consistent with the reasoning. 0 marks: No relevant content Indicative content adoption / gamete donation unsuitable as offspring not biologically theirs natural conception too risky / only 50% chance of healthy offspring natural conception would cause worry whether baby would be healthy or not (therefore) choice is between PGD and PND pros of PGD baby would be theirs results obtained at an early stage high chance baby produced would be healthy parents would have confidence of having a heathy baby from start of
4.6 Inheritance, variation and evolution Reproduction part 2 pregnancy cons of PGD slight / 0.2% chance of misdiagnosed embryo expensive procedure cost to NHS of non-essential procedure (unhealthy) embryos might be destroyed large number of embryos produced so healthy embryos may be destroyed ethical issues of using embryos for research some people are opposed to IVF due to their religious beliefs pros of PND natural conception less invasive for mother psychological benefit of producing child naturally 99% / high chance that result of test will be conclusive cons of PND sampling technique invasive to mother risk of miscarriage risk of infection long wait before test can be carried out 50% chance baby will have allele for Huntington s disease parents will have a difficult decision to make if baby is unheathly baby may be aborted ethical / religious issues of abortion a justified conclusion lower risk of miscarriage compared to PND frozen embryos can be used to have another healthy child PGD occurs before pregnancy / implantation PGD does not involve abortion so less trauma / less pain / ethical comparison spare healthy embryos may be used for research / medical treatment
4.6 Inheritance, variation and evolution Variation and Evolution Explain how the owls in the image may have evolved from a common ancestor to become different species. Use information from the image.
4.6 Inheritance, variation and evolution Variation and Evolution owls have become geographically isolated from each other or arctic ice / temperature in different areas have separated the original population 1 northern area is much colder and has snow / ice allow examples biotic (eg food / predators) or abiotic 1 genetic variation / mutations in each population allow gene(s) / mutation 1 (natural selection occurs so) better adapted survive to reproduce 1 passing on their favourable allele(s) 1 until individuals of the two populations can no longer interbreed (to produce fertile offspring) 1
4.7 Ecology - Adaptation Some animals are adapted to survive in very cold conditions such as the Arctic. Explain how the adaptations of Arctic animals help them to survive in cold conditions.
4.7 Ecology - Adaptation Level 3: Relevant adaptations are identified, given in detail and logically linked to form a clear account. 5-6 Level 2: Relevant adaptations are identified, and there are attempts at logical linking. The resulting account is not fully clear Level 1: Adaptations are identified and stated simply, but their relevance is not clear and there is no attempt at logical linking. No relevant content Indicative content a small SA:V ratio means less thermal energy transferred to surroundings thick fur or hollow hair shafts traps a layer of air which acts as an insulating layer stopping transfer of thermal energy a layer of fat or blubber under the skin acts as an insulating layer or as a food store for respiration when food is in short supply small ears reduces surface area for thermal energy transfer white colour camouflage in the snow so prey do not see them coming and they get more to eat or so predators do not see them and they can escape large feet to spread weight over snow so they can run faster hibernate in winter to conserve energy stores allow heat loss for transfer of thermal energy 3-4 1-2 0
4.7 Ecology Organisation of an ecosystem In one area of the field there is a lot of grass growing in the same area as dandelions. Suggest why the dandelions may not grow well in this area.
4.7 Ecology Organisation of an ecosystem Level 2 (3 4 marks): A detailed and coherent explanation is given. Logical links between clearly identified relevant points are made to explain why dandelion growth may be limited. Level 1 (1 2 marks): Discrete relevant points are made. The logic may be unclear. 0 marks: No relevant content Indicative content factors that may be considered: competition for resources including: light water space mineral ions (allow nutrients / salts / ions from the soil) reference to why growth may be limited: (light) energy for photosynthesis water as a raw material for photosynthesis / support surface area exposed to light sugar / glucose produced in photosynthesis (space) to grow bigger (space) for growth of root system (mineral ions) for growth (mineral ions / sugar) for production of larger molecules or named example
4.7 Ecology - Biodiversity In the last 200 years the concentration of carbon dioxide in the Earth s atmosphere has risen. Explain how a rise in carbon dioxide concentration in the atmosphere can decrease biodiversity.
4.7 Ecology - Biodiversity Level 3 (5 6 marks): A full explanation is given that is coherent and logically structured, linking effect of increase in carbon dioxide to climate change and effects on biodiversity. Level 2 (3 4 marks): An attempt is made to link the effects of rising carbon dioxide levels to climate change and biodiversity. The logic may be inconsistent at times but builds towards a coherent explanation. Level 1 (1 2 marks): Discrete relevant points made. The logic may be unclear and attempts at reasoning may not be consistent. 0 marks: No relevant content. Indicative content rise in carbon dioxide increases atmospheric temperature / causes global warming global warming causes extreme weather patterns such as rise in sea levels increased or decreased rainfall frequency of storms / droughts rise in sea levels means habitats will change due to flooding rise in sea levels could increase salt in soil increased rainfall will increase water levels severity of storms / droughts could affect photosynthesis consequences of changes are loss of or damage to habitats which will affect animal and plant distributions by increasing migration or species dying off which decreases biodiversity
Chemistry Paper 1 5.1 Atomic Structure and the Periodic Table Simple Model The Periodic Table 5.2 Bonding, structure and the Periodic Table Chemical Bonds Structure & Properties Bonding in Carbon 5.3 Quantitative Chemistry Making Salts 5.4 Chemical Changes Reactivity of metals Electrolysis 5.5 Energy Changes Exothermic and endothermic Paper 2 5.6 Rate and extent of chemical change Rates of Reaction 5.7 Organic Chemistry Carbon Compounds 5.8 Chemical Analysis Chromatography 5.9 Chemistry of the atmosphere Development of the atmosphere Global warming & the Greenhouse Effect Impact of atmospheric pollutants 5.10 Using Resources Potable Water Life Cycle Assessments
5.1 Atomic Structure and the Periodic Table Simple Model In 1864, atoms were thought to be particles that could not be divided up into smaller particles. By 1898, the electron had been discovered and the plum pudding model of an atom was proposed. Figure 2 shows the plum pudding model of an atom of carbon and the nuclear model of an atom of carbon. Compare the position of the subatomic particles in the plum pudding model with the nuclear model.
5.1 Atomic Structure and the Periodic Table Simple Model plum pudding model has a single ball of positive charge and nuclear model has positive charges in the centre / nucleus 1 plum pudding model has electrons in random positions and nuclear model has electrons in fixed positions 1 plum pudding model has no nucleus and the nuclear model has a nucleus 1 plum pudding model has no neutrons and the nuclear model has neutrons in the nucleus 1
5.1 Atomic Structure and the Periodic Table The Periodic Table The halogens are in Group 7 of the periodic table. Explain the trend in reactivity of the halogens.
5.1 Atomic Structure and the Periodic Table The Periodic Table Level 3 (5 6 marks): A relevant and coherent explanation of the trend in reactivity. The response makes logical links between the points raised and considers both the number of energy levels and the distance between the nucleus and the outer energy level. Level 2 (3 4 marks): Statements that are linked to provide a simple explanation of the trend in reactivity using either the number of energy levels or the distance between the nucleus and the outer energy level. Level 1 (1 2 marks): Simple statements made about the halogens or the trend in reactivity. 0 marks: No relevant comment Indicative content Simple statements / descriptions have 7 electrons in the outer shell need to gain an electron form ions with a -1 charge halogens further down the group are less reactive (or vice versa) halogens further down the group have more shells or energy levels (or vice versa) Linked statements / explanations have 7 electrons in the outer shell so need to gain an electron to have the electronic structure of a noble gas halogens further down the group are less reactive because they have more shells or energy levels (or vice versa) halogens further down the group have more shells or energy levels so less attractive force on the incoming electron (or vice versa) halogens further down the group have more shells or energy levels so more shielding against the incoming electron (or vice versa) outer electrons of halogens further down group are further away from the attractive force of the nucleus (or vice versa) an electron is less easily gained because there are more shells or energy levels (or vice versa) an electron is less easily gained because the outer electrons are further from the attractive force of the nucleus (or vice versa)
5.2 Bonding Structure and Properties of matter Chemical Bonds The figure below shows the electronic structure of an oxygen atom and a calcium atom. Describe how the calcium atom and the oxygen atom forms calcium oxide. You should give the charge on each ion formed.
5.2 Bonding Structure and Properties of matter Chemical Bonds calcium loses electrons and oxygen gains electrons max 3 for incorrect reference to atom / ion or to oxygen / oxide 1 two electrons are transferred 1 calcium has a 2+charge 1 oxide has a 2 charge 1
5.2 Bonding, Structure and Properties of matter How bonding relates to properties Explain why metals conduct electricity. Refer to structure and bonding in your answer.
5.2 Bonding, Structure and Properties of matter How bonding relates to properties giant structure of atoms 1 delocalised electrons 1 (delocalised electrons) are free to move 1 through the whole structure 1
5.2 Bonding, Structure and Properties of matter Bonding in Carbon Carbon nanotubes are cylindrical fullerenes. Explain the properties of carbon nanotubes. Answer in terms of structure and bonding.
5.2 Bonding, Structure and Properties of matter Bonding in Carbon Level 3 (5 6 marks): A detailed and coherent explanation applying knowledge of the properties of nanotubes, with clear and logical links to reasons why carbon nanotubes have these properties Level 2 (3 4 marks): Description contains relevant statements that demonstrate clear knowledge of the properties of nanotubes. Attempt made to link properties to explanation of why these properties occur, but logic may be unclear Level 1 (1 2 marks): Simple relevant statements of the properties of nanotubes, demonstrating knowledge, but no linking to an explanation of why these properties occur. 0 marks: No relevant content. Indicative content properties: high tensile strength high electrical / thermal conductivity high melting point explanations: nanotubes are fullerenes based on hexagonal rings of carbon atoms which means that each carbon forms three covalent bonds with three other carbon atoms covalent bonds are strong or need a lot of energy to break them so nanotubes are strong / have high tensile strength and have a high melting point the structure means that one electron from each carbon atom is delocalised as in metals and graphite, the delocalised electrons can move throughout the structure allowing the carbon nanotube / fullerene to conduct thermal energy and electricity
5.3 Quantitative Chemistry Making Salts The salt copper sulfate can be made by reacting copper carbonate with dilute sulfuric acid. CuCO3(s) + H2SO4(aq) CuSO4(aq) + H2O(l) + CO2(g) (a) sample of copper You do not need to write a risk assessment or include safety points. Write a method that a student could use to prepare a pure, dry
5.3 Quantitative Chemistry Making Salts Level 3 (5 6 marks): A full, detailed and coherent plan covering all the major steps is provided, which outlines the apparatus required and sets out the steps needed in a logical manner that could be followed by another person to produce a pure, dry sample of copper nitrate. Level 2 (3 4 marks): The substantive content of a plan is present but may be missing some steps. The plan may not be in a completely logical sequence but leads towards the production of a pure, dry sample of copper nitrate. Level 1 (1 2 marks): Simple statements relating to relevant apparatus or steps are made but they may not be in a logical order. The plan would not allow another person to produce the sample. 0 marks: No relevant content Indicative content pour a suitable volume of nitric acid into a suitable container add a small amount of copper carbonate to the acid and stir until the effervescence stops continue to add small amounts of copper carbonate to the acid and each time stir until any effervescence stops eventually when there is no reaction / effervescence when the copper carbonate is added filter the mixture to remove the excess copper carbonate pour the filtrate (copper nitrate solution) into an evaporating basin and heat to evaporate a small amount of the water leave the copper nitrate solution to crystallise remove the crystals from the solution remaining and dry the crystals
5.4 Chemical Changes Reactivity of metals, reactions of acids Magnesium reacts with hydrochloric acid to produce magnesium chloride and hydrogen. Plan an investigation to find the accurate volume of hydrogen produced from magnesium. You do not need to write about safety precautions.
5.4 Chemical Changes Reactivity of metals, reactions of acids Level 2: The plan would lead to the production of a valid outcome. All key steps are identified and logically sequenced. 4-6 Level 1: The plan would not lead to a valid outcome. Some relevant steps are identified, but links are not made clear. 1-3 No relevant content 0 Indicative content an ideal plan would be: use a rule to measure the length / use a balance to find the mass of the piece of magnesium put magnesium into conical flask use measuring cylinder for dilute hydrochloric acid add dilute hydrochloric acid to conical flask connect bung (& delivery tube) into conical flask measuring cylinder is filled with water and inverted / upside down in bowl of water OR uses a gas syringe hydrogen flows through a delivery / rubber tube into measuring cylinder wait until all magnesium reacts / use excess dilute hydrochloric acid record volume when bubbles stop other things they could mention: use accurate / 2 dp balance to collect gas use measuring cylinder / gas syringe with best resolution add bung quickly to ensure no gas escapes gas is collected in graduated apparatus (not test tube) repeat experiment (with same length / mass of magnesium) repeat at same temperature since volume of gas will be different
5.4 Chemical Change - Electrolysis A student investigated the electrolysis of different concentrations of sodium chloride solution. Figure 1 shows the apparatus used. Explain how chlorine gas is produced at the positive electrode.
5.4 Chemical Change - Electrolysis chloride ions are negatively charged 1 (and so are) attracted to the positive electrode 1 (at the electrode) the chloride ions lose electrons allow chloride ions are oxidised 1 to form chlorine atoms / molecules allow instead of last two marking points an answer of 2Cl Cl2+ 2e for 2 marks 1
5.5 Energy Changes Exothermic and endothermic Some students investigated the reactivity of four unknown metals, W, X, Y and Z. The letters are not the symbols of these elements. The students used metal salt solutions of copper nitrate, magnesium sulfate and zinc chloride. This is the method used. 1. Pour a solution of a metal salt into a glass beaker. 2. Measure the temperature of the solution. 3. Add 1 g of metal to the solution. 4. Measure the temperature of the solution. 5. Calculate the temperature increase. The students did the experiment using each salt solution with each metal. One student said that the investigation was not valid (a fair test). Write a plan for the investigation that includes improvements to the method and apparatus.
5.5 Energy Changes Exothermic and endothermic Level 2 (3 4 marks): A detailed and coherent plan covering all the steps. The steps include the improvements and are set out in a logical manner. Level 1 (1 2 marks): Simple statements of improvements to the apparatus or steps are made but they may not be set out in a logical manner. 0 marks: No relevant content Indicative content Simple statements stir the solution use the same amount of each solution use the same concentration of solution put insulation or a lid on the beaker measure how high temperature goes Coherent statements in a logical order pour a fixed, measured volume of the metal salt solution into a plastic / polystyrene cup measure and record the temperature of the solution stir and add 1 g of metal to the solution (put a lid on the cup) measure and record the temperature after a set time or measure and record the greatest / highest temperature calculate and record the temperature increase (repeat each individual experiment at least two more times and calculate the mean temperature increase)
5.6 Rate and extent of chemical change Rate of Reaction A student investigated the rate of the reaction between magnesium and dilute hydrochloric acid. The student used the apparatus shown in Figure 1 to collect the gas produced. Outline a plan to investigate how the rate of this reaction changed when the concentration of the hydrochloric acid was changed. Describe how you would do the investigation and the measurements you would make. Describe how you would make it a fair test. You do not need to write about safety precautions.
5.6 Rate and extent of chemical change Rate of Reaction Level 3 (5 6 marks): A coherent method is described with relevant detail, which demonstrates a broad understanding of the relevant scientific techniques and procedures. The steps in the method are logically ordered with the dependent and control variables correctly identified. The method would lead to the production of valid results. Level 2 (3 4 marks): The bulk of a method is described with mostly relevant detail, which demonstrates a reasonable understanding of the relevant scientific techniques and procedures. The method may not be in a completely logical sequence and may be missing some detail. Level 1 (1 2 marks): Simple statements are made which demonstrate some understanding of some of the relevant scientific techniques and procedures. The response may lack a logical structure and would not lead to the production of valid results. 0 marks: No relevant content Indicative content remove bung and add magnesium start stopclock / timer measure volume of gas at fixed time intervals repeat with different concentrations of acid control volume of acid control initial temperature of acid control amount / mass / length / particle size of magnesium
5.7 Organic Chemistry Carbon Compounds The diagram shows the separation of crude oil in a fractionating column. Explain how crude oil is separated into different fractions by fractional distillation.