Insights into Nuclear Core Dynamics and Structure
Exploring the dynamics of nuclear cores, this research delves into the intricacies of probing the NN repulsive core, modern NN potentials, and field theories of nucleons and mesons. The study encompasses the isospin dependence, non-nucleonic components, hidden color, and gluons within the nucleus, s
1 views • 63 slides
Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Describing the Most Disgusting Room Imaginable
Imagine a room so vile and repulsive, with clothes scattered like wrestlers, damp socks emitting a foul stench, and toys in disarray with Barbie dolls in twisted scenes. The air thick with neglect, each corner a reminder of chaos and filth that defy any sense of order or hygiene. A place where clean
1 views • 7 slides
Investigation of Short-Range Correlations in Heavy Ion Collisions
Short-range correlations in nuclei play a crucial role in understanding the properties of nuclear wave functions and the transition from baryonic to quark-gluon degrees of freedom. Early theoretical work emphasized the importance of short-range correlations, particularly in modeling the nucleon spec
0 views • 17 slides
Weak Interactions and Hydrogen Bonding in Molecular Forces
Exploring van der Waals forces, hydrogen bonding, and weak interactions in intermolecular forces and surface interactions. Understanding interactions between backbone peptide groups and orientation dependence of hydrogen bonding through dispersion forces and repulsive potentials.
0 views • 22 slides
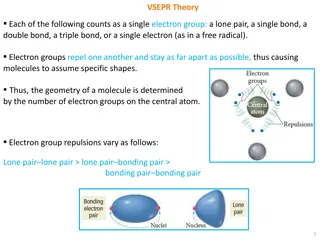
VSEPR Theory for Molecular Geometry
VSEPR theory explains how the arrangement of electron groups around a central atom determines the shape of molecules based on the repulsions between different types of electron groups. The geometry of a molecule is influenced by factors such as lone pairs, single, double, or triple bonds, and their
1 views • 16 slides
Strongly Interacting Quantum Particles in One Dimension - Research at Aarhus Universitet
Researchers at Aarhus Universitet delved into the dynamics of confined few-body quantum systems in one dimension, focusing on the interactions and properties of strongly interacting particles. Through experimental realizations and theoretical models, they explored the behavior of bosons and fermions
0 views • 22 slides
States of Matter
In this lecture by Assistant Prof. Dr. Fouad Al-Saady on physical pharmacy, the focus is on understanding the intermolecular forces that govern the behavior of molecules in different states of matter. Topics covered include cohesive and adhesive forces, repulsive and attractive forces, as well as th
0 views • 27 slides
Essential Magnetism Concepts for On-Level Class
Magnetism is a fascinating physical phenomenon arising from the motion of electric charge, leading to attractive and repulsive forces between objects. Explore key concepts like magnetic fields, magnetic domains, magnetosphere, magnetic induction, and metals in this informative material.
0 views • 17 slides
Introduction to Coulomb's Law in Electrostatics
Discover the principles of electrostatics through Coulomb's Law. Learn about the relationship between charges, forces, and distances in electrical interactions. Explore examples illustrating the attractive and repulsive forces between charged objects.
0 views • 8 slides
Short-Range Correlations in Heavy Ion Collisions
Short Range Correlations in Nuclei Fluctuations of close proximity nucleon pairs. Small center of mass momentum, large relative momentum, important in investigation of large relative momentum and short distance properties of nuclear wave functions. Unexplored dynamics of nuclear repulsive core and i
0 views • 22 slides
Magnetism: Forces, Fields, and Phenomena
Discover the fascinating world of magnetism with insights into magnetic forces, fields, and phenomena. Uncover the mysteries behind magnetic domains and the Earth's magnetosphere. Dive into the science of magnetic materials and their influence on objects in the world around us. Gain a deeper underst
0 views • 17 slides