Crystal Field Theory in Transition Metal Complexes
Crystal Field Theory (CFT) explains the colors and magnetic properties of transition metal complexes. It focuses on the energy changes in d-orbitals of metal ions caused by surrounding ligands. This theory, developed in 1929, provides insights into the bonding interactions in complex compounds. The
10 views • 44 slides
Unsaturated Hydrocarbons Alkynes
Chapter three delves into the world of alkynes, focusing on their structure, hybridization, bonding, nomenclature, physical properties, preparation, and reactions. Alkynes are hydrocarbons with at least one triple bond, forming a homologous series with the molecular formula CnH2n-2. The sp hybridiza
0 views • 20 slides
Exploring Quantum Theory and the Atom: Electrons in Atoms and the Periodic Table
Delve into the fascinating world of quantum theory and the atom in Chapter 9, where we compare Bohr's model with the quantum mechanical model. Understand de Broglie's wave-particle duality and Heisenberg's uncertainty principle's impact on our current electron view. Discover the relationships among
0 views • 31 slides
Armed Intruder Tabletop Exercise - Preparedness and Coordination
Enhance preparedness for armed intruder situations in schools through a tabletop exercise focusing on testing response plans, coordination with partners, and identifying areas for improvement. The exercise aims to strengthen relationships with response partners and improve emergency communication st
0 views • 35 slides
Understanding Hybridization in Organic Chemistry
Delve into the complexities of the Lewis octet model and the insights provided by Linus Pauling's localized valence bond hybridization model to explain bond shapes in molecules, reactivity trends, and electron distribution in double and triple bonds. Discover how hybridization transforms atomic orbi
0 views • 22 slides
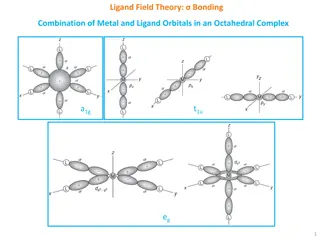
Understanding Ligand Field Theory in Octahedral Complexes
Ligand Field Theory explains the bonding interactions between metal and ligand orbitals in octahedral complexes. This theory involves the combination of metal and ligand orbitals to form molecular orbitals, leading to specific electronic configurations. The overlap of metal and ligand group orbitals
1 views • 10 slides
Understanding Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Cyber Threat Detection and Network Security Strategies
Threat detection is crucial in analyzing security ecosystems to identify and neutralize malicious activities. Methods like leveraging threat intelligence, behavior analytics, setting intruder traps, and conducting threat hunts are essential for proactive security. Implementing security through obscu
1 views • 51 slides
Understanding Valence Bond Theory in Chemistry
Valence Bond Theory (VBT) explains the formation of covalent bonds through overlapping of valence orbitals, introducing Sigma and Pi bonds. This theory is essential to understand the geometry and stability of complex molecules.
1 views • 19 slides
Understanding Organic Chemistry: Introduction to Carbon Compounds
Organic chemistry is the study of carbon compounds, with carbon's unique electronic structure allowing for a vast array of compounds. This field touches many aspects of life, from medicines to polymers. The nucleus of an atom, comprising protons and neutrons, is surrounded by electrons occupying orb
1 views • 5 slides
Understanding Covalent Bonds and Molecular Structure in Organic Chemistry
The neutral collection of atoms in molecules held together by covalent bonds is crucial in organic chemistry. Various structures like Lewis and Kekulé help represent bond formations. The concept of hybridization explains how carbon forms tetrahedral bonds in molecules like methane. SP3 hybrid orbit
0 views • 4 slides
Understanding Different Types of Chemical Bonds
Metallic bonds involve atoms giving up valence electrons to form an electron sea, covalent bonds entail electron sharing to fill outer orbitals, ionic bonds form when atoms with different electronegativities attract, Van der Waals bonds include London forces between atoms, and hydrogen bonds occur i
0 views • 6 slides
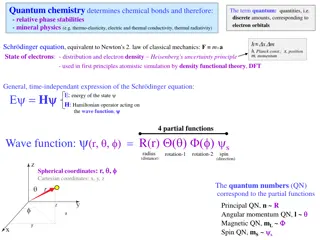
Understanding Quantum Chemistry and Electron Orbitals
Quantum chemistry plays a key role in determining chemical bonds, phase stabilities, and mineral physics through the study of electron orbitals, quantum numbers, and energy levels. This involves concepts such as the Schrödinger equation, quantum quantities, and the uncertainty principle. The arrang
0 views • 31 slides
Best Wireless Alarms in Leixlip
Are you looking for the Best Wireless Alarms in Leixlip? Then contact Covert Security. They offer comprehensive residential and commercial security services nationwide. They specialize in providing bespoke intruder wireless alarms and CCTV packages t
0 views • 6 slides
Understanding Alkenes: Structure, Bonding, and Naming
Alkenes are unsaturated hydrocarbons containing a C=C double bond. This chapter covers topics such as alkene structure, hybridization, naming, isomerization, physical properties, preparation methods, stability rules, and addition reactions. Learn about the sp2 hybridization of orbitals in alkenes, o
0 views • 38 slides
Understanding Angular Overlap Method in Advanced Inorganic Chemistry
Exploring the Angular Overlap Method (AOM) in advanced inorganic chemistry provides a qualitative discussion on the physical rationale behind the theory of complexes. By considering the interaction of atomic orbitals and the degree of overlap, AOM offers insights into energy quantification in coordi
0 views • 15 slides
Understanding Atomic Orbitals and Electron Arrangement
Learn about the properties of atomic orbitals and how they determine the distance from the nucleus and the shape of orbitals. Explore the main energy levels, sublevels, and the arrangement of electrons following the Aufbau Principle within an atom.
4 views • 28 slides
Understanding Alkynes in Organic Chemistry
Alkynes are unsaturated hydrocarbons with at least one triple bond, following a molecular formula of CnH2n-2. This group of compounds is discussed in Chapter three, covering topics like structure, hybridization, common naming, physical properties, preparation, and reactions. The sp hybridization of
1 views • 20 slides
Understanding Density Functional Theory in Chemistry
Density Functional Theory (DFT) plays a crucial role in chemistry by uniquely determining molecular properties based on electron density. The Hohenberg-Kohn Theorem establishes the foundation, with the goal of finding an exact energy functional expressed in terms of density. Various concepts like th
0 views • 19 slides
Understanding Atomic Structure and Interatomic Bonding
Atomic structure is defined by the atomic number (Z) and atomic mass (A). Quantum mechanics governs atomic and subatomic particles, introducing discrete energy levels. The Bohr atomic model describes electrons orbiting the nucleus in defined orbitals. Quantum numbers characterize electron properties
0 views • 15 slides
If you are looking for CCTV in Malahide
If you are looking for CCTV in Malahide, Angel Alarms, based in Yellow Walls, Dublin, is a premier provider of top-notch security solutions designed to protect your home and business. We specialise in the installation of intruder alarms, burglar alar
0 views • 6 slides
Insights into Seniority Isomers in Nuclear Physics
Explore the concept of seniority isomers in nuclear physics, delving into topics like semi-magic isomers, alignment properties of intruder orbitals, and the role of seniority mixing. Discover the significance of Z=50, N=82 isomers and the structural properties associated with them. Uncover the essen
0 views • 21 slides
Understanding Electron Configuration and Quantum Numbers in Chemistry
Explore the concept of electron configurations, quantum numbers, and orbital filling rules in chemistry. Discover the principles governing the arrangement of electrons in atoms, including the Aufbau Principle, Pauli Exclusion Principle, and Hund's Rule. Gain insight into orbital energy levels and th
0 views • 18 slides
Quantum Mechanics in Chemistry Lecture 1 Overview
Explore the fundamentals of quantum mechanics in chemistry, focusing on electron behavior, orbital solutions, bonding, and interactions. Learn about the role of different orbitals, resonance, and orbital mixing in the Schrödinger equation to understand molecular structure and behavior.
0 views • 24 slides
Crystal Field Theory and Color Exhibited by Coordination Compounds
Crystal Field Theory (CFT) explains the colors exhibited by coordination compounds based on the absorption of light and electron transitions in d-orbitals. The theory describes how ligands interact with transition metal ions, causing the d-orbitals to split in energy levels. This split results in th
0 views • 30 slides
Optimizing Barrier Coverage Quality in Wireless Sensor Networks
Explore the optimization of barrier coverage quality in wireless sensor networks, focusing on maximizing intruder detection, detection probability, and minimizing data transmission time. The problem is modeled graphically and solved using the Minimum Cost Maximum Flow Algorithm, with a background in
1 views • 37 slides
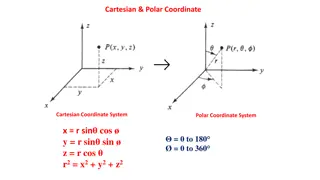
Understanding Cartesian and Polar Coordinate Systems
Explore the concepts of Cartesian and Polar coordinate systems, including their formulas and visual representations. Dive into the relationships between Cartesian and Polar coordinates, as well as their applications in mathematics and physics. Discover orbital shapes such as Px, Py, Pz, and dz2 orbi
0 views • 12 slides
Hospital Violent Intruder Tabletop Exercise Program
This exercise program, developed by the Missouri Hospital Association, focuses on hospital preparedness for violent intruder incidents. It addresses the increasing rates of crime in healthcare institutions, including assaults, rapes, and homicides. The tabletop exercise aims to enhance the hospital'
0 views • 45 slides
Understanding Ultraviolet and Visible Spectroscopy in Chemistry
Ultraviolet and visible spectroscopy in chemistry involves the absorption of light energy by molecules, dependent on their electronic structure. This process, also known as electronic spectrum, entails energy transitions of electrons in molecular orbitals. The region of the electronic spectrum inclu
0 views • 29 slides
Understanding Atomic Orbitals: Counting, Subshells, Energies, and Electrons
Learn about the basics of atomic orbitals, including the counting of orbitals in shells and subshells, the distribution of electrons in different energy levels, and the symmetrical nature of orbital labeling. Dive into the rules governing electron placement based on quantum mechanics and explore the
0 views • 6 slides
Understanding Colour in Organic Molecules through Conjugated Systems
Conjugated systems in organic molecules exhibit alternating single and double bonds, leading to unique molecular orbitals and absorption patterns. With increased conjugation, molecules can absorb visible light, producing vibrant colors like the red from lycopene in tomatoes.
0 views • 28 slides
Update on NRC Proposed Rule to Amend 10 CFR Part 61 (Sept. 2014)
The NRC has proposed rule amendments for 10 CFR Part 61 in September 2014, focusing on licensing requirements for land disposal of LLRW and performance objectives. The update covers new staff requirements, including period of performance tiers, intruder assessment, defense-in-depth, and more. These
0 views • 9 slides
General Chemistry Homework Questions
Solve quantum number problems, determine total orbitals, electron configurations, isoelectronic series, atomic radius order, and ionization energies in this set of General Chemistry questions.
0 views • 10 slides
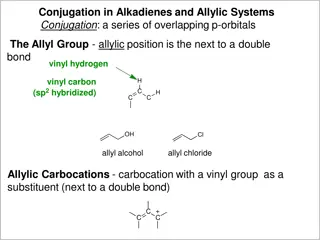
Understanding Allylic Systems and Reactions
Conjugation in alkadienes and allylic systems involves overlapping p-orbitals, with the allyl group featuring resonance-stabilized allylic carbocations. Allylic halides exhibit enhanced reactivity in SN1 and SN2 reactions, and allylic free radicals demonstrate distinct electron density patterns. Add
0 views • 25 slides