Quantum Mechanics in Chemistry Lecture 1 Overview
Explore the fundamentals of quantum mechanics in chemistry, focusing on electron behavior, orbital solutions, bonding, and interactions. Learn about the role of different orbitals, resonance, and orbital mixing in the Schrödinger equation to understand molecular structure and behavior.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
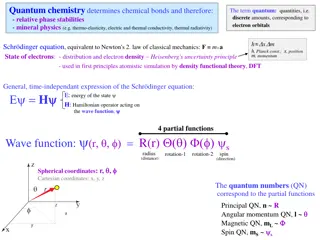
Chemistry 2 Lecture 1 Quantum Mechanics in Chemistry
Your lecturers 8am 12pm Asaph Widmer-Cooper Adam Bridgeman Room 316 Room 543A asaph.widmer-cooper@sydney.edu.au adam.bridgeman@sydney.edu.au
Revision H2+ Near each nucleus, electron should behave as a 1s electron. At dissociation, 1s orbital will be exact solution at each nucleus r
Revision H2+ At equilibrium, we have to make the lowest energy possible using the 1s functions available ? r r
Revision H2+ = 1sA 1sB anti-bonding 1sA 1sB E 1sB 1sA = 1sA + 1sB bonding 1sA 1sB
Revision H2 = 1sA 1sB anti-bonding 1sA 1sB E 1sB 1sA = 1sA + 1sB bonding 1sA 1sB
Revision He2 = 1sA 1sB anti-bonding 1sA 1sB E 1sB 1sA = 1sA + 1sB bonding 1sA 1sB NOT BOUND!!
2nd row homonuclear diatomics Now what do we do? So many orbitals! 2p 2p 2s 2s 1s 1s
Interacting orbitals Orbitals can interact and combine to make new approximate solutions to the Schr dinger equation. There are two considerations: 1.Orbitals interact inversely proportionally to their energy difference. Orbitals of the same energy interact completely, yielding completely mixed linear combinations. In quantum mechanics, energy and frequency are related (E=h ). So, energy matching is equivalent to the phenomenon of resonance. 2.The extent of orbital mixing is given by the resonance integral . We will show how beta is calculated in a later lecture.
Interacting orbitals 1. Orbitals interact proportionally to the inverse of their energy difference. Orbitals of the same energy interact completely, yielding completely mixed linear combinations. 1 ( ) = 2 2 sA sB 2 2p 2p 2s 2s 1 ( ) = + 2 sB 2 sA 2 2 sA sB 2 1s 1s
(First year) MO diagram Orbitals interact most with the corresponding orbital on the other atom to make perfectly mixed linear combinations. (we ignore core). 2p 2p 2s 2s
Molecular Orbital Theory - Revision Can predict bond strengths qualitatively 2 p * 2 p * p 2 p 2 2 s * N2 Bond Order = 3 diamagnetic s 2
Interacting orbitals 1. The extent of orbital mixing is given by the integral = something 2p 2p 2s 2s s p 2 2 The 2s orbital on one atom can interact with the 2p from the other atom, but since they have different energies this is a smaller interaction than the 2s-2s interaction. We will deal with this later. 1s 1s
Interacting orbitals 1. The extent of orbital mixing is given by the integral = 0 cancels 2p 2p 2s 2s s p 2 2 There is no net interaction between these orbitals. The positive-positive term is cancelled by the positive-negative term 1s 1s
More refined MO diagram orbitals can now interact 2 p * 2 p * p 2 p 2 = something 2 s * s p 2 2 s 2
More refined MO diagram orbitals can interact * 2 p * 2 p * p 2 p 2 2 s * * s 2
More refined MO diagram orbitals do not interact * 2 p * 2 p * * p 2 p 2 2 s * * s 2
More refined MO diagram sp mixing * 2 p * 2 p * * p 2 p 2 This new interaction energy Depends on and the energy spacing between the 2s and the 2p 2 s * * s 2
sp mixing Largest energy gap, and thus smallest mixing between 2s and 2p is for Fluorine. Smallest energy gap, and thus largest mixing between 2s and 2p is for Boron. 2p 2 Z = En c.f. 2 n 2s
sp mixing * * * * * * * * * * * * diamagnetic weakly bound paramagnetic Be2 B2 C2 N2
Learning outcomes Use the principle that the mixing between orbitals depends on the energy difference, and the resonance integral, . Apply the separation of and bonding to describe electronic structure in simple organic molecules. Rationalize differences in orbital energy levels of diatomic molecules in terms of s-p mixing.
Next lecture Particle in a box approximation solving the Schr dinger equation. Week 10 tutorials Wavefunctions and the Schr dinger equation.
Practice Questions 1. 2. Why is s-p mixing more important in Li2 than in F2? How many core, -bonding, and -electrons are there in a) acetylene b) ethylene c) benzene d) buckminsterfullerene Check that your total number of electrons agrees with what is expected (6 per carbon, 1 per hydrogen).