Section 5.6 — Intermolecular Forces & Properties
Water's exceptional properties, including polarity and hydrogen bonding, make it essential for life and an excellent solvent. The intermolecular forces in water allow it to function as a vital molecule with distinct characteristics that enable it to interact with other substances like soap. By lever
1 views • 23 slides
Understanding Virus-Cell Interactions: Mechanisms and Consequences
Viruses interact with host cells in various ways, encoding genes that manipulate cell functions for their benefit. These interactions can range from benign to lethal outcomes. Factors influencing these interactions include viral factors, cellular responses, and the presence of virulence factors. Dif
0 views • 37 slides
Exploring Particles and Fundamental Interactions in the Universe
Delve into the intricate world of particles and fundamental interactions in the Universe as explained by Professor Emeritus George Lazarides from Aristotle University of Thessaloniki. Discover the structure of matter, classification of particles based on interactions, constituents of hadrons, conser
1 views • 36 slides
Understanding Hyperfine Interactions in Atomic Physics
Hyperfine interactions play a crucial role in atomic physics, leading to small energy shifts and splitting of degenerate levels in atoms and molecules. These interactions involve the electromagnetic multipole interactions between the nucleus and electron clouds, resulting in the splitting of energy
13 views • 154 slides
Role of Chemistry in Pharmaceutical Sciences
Medicines and drugs used for treating diseases are organic or inorganic chemicals. Studying drug chemistry is essential for pharmacists. This course covers fundamentals of chemistry, states of matter, radiochemistry, thermodynamics, kinetics, and chemical equilibria. Important subtopics include atom
2 views • 35 slides
INTERMOLECULAR FORCES (IMF)
Exploring the concepts of intramolecular bonds versus intermolecular forces, the impact of intermolecular forces on states of matter, different types of intermolecular forces, and the significance of dipoles in chemistry. Dive into the world of IMF with visual aids and real-world analogies.
2 views • 12 slides
5 Medical Cannabis Interactions Every Physician Must Understand
As medical cannabis becomes increasingly integrated into healthcare practices, itu2019s imperative for physicians to be well-versed in potential interactions with other medications. While cannabis offers promising therapeutic benefits, its interactions with certain drugs can pose risks to patients.
1 views • 2 slides
Exploring Supramolecular Chemistry: Insights and Applications
Supramolecular chemistry delves into the chemistry of molecular assemblies, intermolecular bonds, and non-covalent interactions, leading to the formation of supra-molecules through aggregation of molecular subunits. Concepts like molecular recognition, self-organization, and host-guest chemistry pla
0 views • 18 slides
Exploring Influences on Consciousness Through Neocortical Interactions
Delve into the intriguing realm of consciousness with Lester Ingber's research on the influences stemming from multiple scales of neocortical interactions. The investigations cover various aspects such as mind over matter, recursive interactions, neuronal scales in the neocortex, and statistical mec
1 views • 41 slides
Understanding Column Chromatography: Methodology, Advantages, Disadvantages, and Applications
Column chromatography, a type of adsorption chromatography, involves separating components based on their affinity to an adsorbent. The methodology includes passing a solvent through a column to improve separation, obtaining a chromatogram, and eluting components for analysis. The principle relies o
2 views • 24 slides
Understanding Van der Waals Forces and Intermolecular Interactions
Van der Waals forces encompass London dispersion forces, dipole-dipole forces, and hydrogen bonding, influencing interactions between atoms and molecules. London dispersion forces are the weakest and present in all molecules, dipole-dipole forces involve permanent dipoles, and hydrogen bonding, the
0 views • 9 slides
Understanding Stereochemistry: Isomers and Their Properties
Stereochemistry explores the fascinating world of isomers, including stereoisomers, geometric isomers, and structural isomers. Stereoisomers have the same molecular formula but differ in spatial arrangement, while geometric isomers lack free rotation around bonds. Structural isomers like dimethyl et
0 views • 27 slides
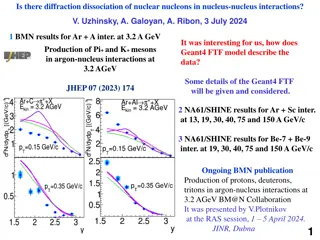
Understanding Diffraction Processes and Meson Production in Nuclear Interactions
Exploration of diffraction dissociation of nuclear nucleons in nucleus-nucleus interactions using Geant4 FTF model and NA61/SHINE results for various nucleus combinations. Insights into meson production in argon-nucleus interactions at different energies and the impact of models like DCM/AGT, UrQMD,
0 views • 17 slides
Understanding Distillation: Methods and Factors Affecting Boiling Points
Distillation is a process used to separate liquids based on their boiling points, with methods like simple, fractional, vacuum, and steam distillation. The boiling point of a compound is influenced by factors such as molecular weight and functional groups, where higher weights and polar groups lead
1 views • 15 slides
Understanding Distillation: Boiling Points and Factors
Distillation is a process of vaporizing and condensing liquids to separate components based on boiling points. This method utilizes differences in boiling points of liquid mixtures to achieve separation. Factors influencing boiling points include molecular weight, functional groups, and intermolecul
0 views • 16 slides
Exploring Latent Heat of Vaporisation through Demonstration
Students will learn about latent heat of fusion and vaporisation, specifically focusing on calculating the latent heat of vaporisation for water. Through a hands-on demonstration, students will understand the concept that a liquid cannot exceed its boiling point temperature, as energy is used to bre
0 views • 4 slides
Understanding Real Gas Behavior in Chemistry
Real gases deviate from ideal gas behavior due to factors like finite volume of gas particles and intermolecular attractions. At high pressures and low temperatures, real gases don't follow ideal gas laws, impacting their molar volume and pressure calculations. This deviation is crucial in analyzing
1 views • 11 slides
PuReMD Design - Initialization, Interactions, and Experimental Results
PuReMD Design involves the initialization of neighbor lists, bond lists, hydrogen bond lists, and coefficients of QEq matrix for bonded interactions. It also implements non-bonded interactions such as charge equilibration, Coulomb's forces, and Van der Waals forces. The process includes the generati
0 views • 23 slides
Understanding Epistasis: Genetic Interactions and Their Implications
Epistasis is a phenomenon where the phenotypic expression of one gene is influenced by interactions with another gene. This concept, first introduced in 1909, plays a crucial role in genetics, affecting various traits and evolutionary processes. The difference between dominance and epistasis lies in
0 views • 41 slides
Understanding UML Sequence Diagrams
UML Sequence Diagrams illustrate high-level interactions between class instances in software programs. They represent method calls and interactions among objects during program execution. The diagrams show interactions for specific circumstances like startup or button clicks. Each class/object is re
0 views • 8 slides
Understanding Intermolecular Forces: Strength, Types, and Examples
Intermolecular forces are attractions between molecules, weaker than chemical bonds. They include London dispersion forces, dipole-dipole interactions, and hydrogen bonding. Strength varies, with covalent bonds being the strongest and London dispersion forces the weakest. Different types of intermol
0 views • 15 slides
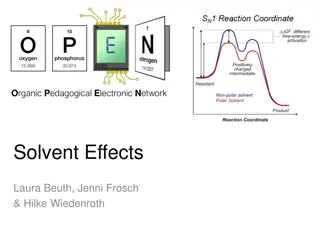
Understanding Solvent Effects in Organic Chemistry
Different solvents can have varying effects on chemical reactions by influencing solubility, stability, and reaction rates. Solvents can control the pathway towards forming either thermodynamic or kinetic products based on their selection. Solutes dissolve based on intermolecular interactions with s
0 views • 6 slides
Chemistry 222 Exam II Review: Gas Laws, Reactions, and Intermolecular Forces
Explore questions related to gas volume changes with pressure, gas density comparisons, stoichiometry in gas reactions, gas velocity comparisons, pressure comparison between gases, boiling point predictions based on intermolecular forces, and ranking molecules based on intermolecular forces.
0 views • 28 slides
Comparison of Models of Nucleus-Nucleus Interactions in CORSIKA
Introduction to the study on models of hadronic interactions at high energies implemented in CORSIKA, a simulation tool used to analyze cosmic ray interactions with Earth's atmosphere. The study compares four widely used models, detailing their features and variants in simulation parameters. Results
0 views • 10 slides
Population Interactions in Nature: Competitive and Cooperative Interactions
Every population, whether animal or plant, engages in competitive and cooperative interactions to fulfill their needs for food, shelter, and resources. Intraspecific competition is common among individuals of the same species, leading to a struggle for survival. Interspecific interactions also play
0 views • 17 slides
Weak Interactions and Hydrogen Bonding in Molecular Forces
Exploring van der Waals forces, hydrogen bonding, and weak interactions in intermolecular forces and surface interactions. Understanding interactions between backbone peptide groups and orientation dependence of hydrogen bonding through dispersion forces and repulsive potentials.
0 views • 22 slides
Understanding Intermolecular Forces in Chemistry
Interactions between static charge distributions in chemistry are governed by Coulomb's Law, playing a crucial role in understanding intermolecular forces. From ion-ion interactions to charge-dipole and charge-quadrupole interactions, the strength of forces varies based on factors like distance and
0 views • 21 slides
Understanding Intermolecular Forces and Dispersion Forces in Molecules
Particle diagrams of liquids, solids, and gases reflect distinct arrangements due to intermolecular forces. The existence of substances as gases, liquids, or solids at room temperature is attributed to the forces between molecules known as intermolecular forces (IMF), with dispersion forces being th
0 views • 30 slides
Understanding Use Cases and Actors in System Design
Explore the concept of use cases in system design, including user goals versus interactions, system boundaries, actors, and how they all come together in use case diagrams. Learn how use cases capture user-visible functions, achieve discrete goals, and represent the interactions between actors and t
0 views • 20 slides
Exploring Intermolecular Bonds and Relative Strengths
Understanding the types of intermolecular bonds - covalent bonding, dispersion forces, dipole-dipole attraction, and hydrogen bonding, along with their relative strengths and factors determining bond strength. Learn about permanent dipole-dipole forces and hydrogen bonding, crucial for phenomena lik
0 views • 18 slides
Understanding the Nature and Characteristics of Matter
Matter is anything with mass and volume, categorized as solid, liquid, or gas based on physical properties. It consists of tiny particles with intermolecular spaces that are in constant motion and have the ability to diffuse and attract each other. Diffusion and osmosis are processes highlighting mo
0 views • 25 slides
Understanding Boiling Points and Intermolecular Forces
Exploring the relationship between intermolecular forces and boiling points, this content discusses trends and anomalies in boiling points of halogens, isomers with the same molecular formula, molecules with similar Mr, and polar molecules. It explains how molecular size, structure, and interactions
0 views • 5 slides
Understanding Population Interactions in Communities
Communities are made up of populations of organisms that interact in various ways, shaping the structure of the community. Population interactions include predator-prey relationships, symbiotic interactions like mutualism and commensalism, parasitism, and competitive exclusion. These interactions in
0 views • 25 slides
Exploring Atomic Dimensions: Scanning Probe Microscopy
Delve into the world of nanoscale imaging with Scanning Probe Microscopy (SPM) techniques like Scanning Tunneling Microscopy (STM) and Atomic Force Microscope (AFM). Unlike optical microscopes, SPM methods break the diffraction limit by relying on intermolecular forces and quantum tunneling for unpa
0 views • 26 slides
Quantum Interactions: Electrons, Phonons, and Hubbard Interaction
Exploring the complexities of electron-electron and electron-phonon interactions, nonequilibrium Green's functions, Hartree-Fock method, Coulomb's law, quantum operator forms, Hubbard interaction, and electron-phonon interactions from first principles. The interactions delve into the behavior of cha
0 views • 20 slides
Understanding Colligative Properties in Chemistry
Colligative properties in chemistry depend on the amount and type of solute particles added to a sample, as well as the intermolecular forces at play. They include vapor pressure, boiling point elevation, and freezing point depression. Vapor pressure is the pressure exerted by a vapor on its surroun
0 views • 7 slides
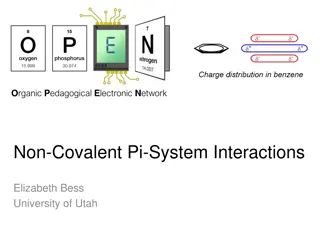
Understanding Non-Covalent Pi-System Interactions in Molecular Structures
Non-covalent interactions play a crucial role in chemical selectivity and molecular recognition. This article discusses the significance of Pi-system interactions, including Pi-Pi and Cation-Pi interactions, in stabilizing molecular structures like DNA helices and G-quadruplexes. Insights into molec
0 views • 6 slides
Understanding Properties of Solutions: A Comprehensive Overview
Solutions are homogeneous mixtures of pure substances where the solute is uniformly dispersed in the solvent. This chapter delves into the formation of solutions, types of solutions (saturated, unsaturated, supersaturated), Henry's Law, the influence of temperature on solubility, and ways of express
0 views • 25 slides
Understanding Heat Transfer in Phase Changes of Water
Water molecules exhibit different behaviors in the liquid and gaseous states due to varying attractions between molecules. To change liquid water to a gas, energy must be added to overcome intermolecular forces, making this process endothermic. The heat absorbed during melting is equal to the heat r
0 views • 22 slides
Genetic Interactions in Chicken Combs: Epistatic and Non-Epistatic Examples
In chicken genetics, epistatic and non-epistatic interactions play a crucial role in determining comb size and shape. Bateson and Punnett's studies on fowls revealed how different gene pairs interact to produce distinctive phenotypes. Epistatic interactions, such as dominant epistasis, influence the
0 views • 16 slides