Exploring Latent Heat of Vaporisation through Demonstration
Students will learn about latent heat of fusion and vaporisation, specifically focusing on calculating the latent heat of vaporisation for water. Through a hands-on demonstration, students will understand the concept that a liquid cannot exceed its boiling point temperature, as energy is used to break intermolecular forces during the phase change. The method involves using specific heat capacity to calculate the latent heat of vaporisation in joules per kilogram.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
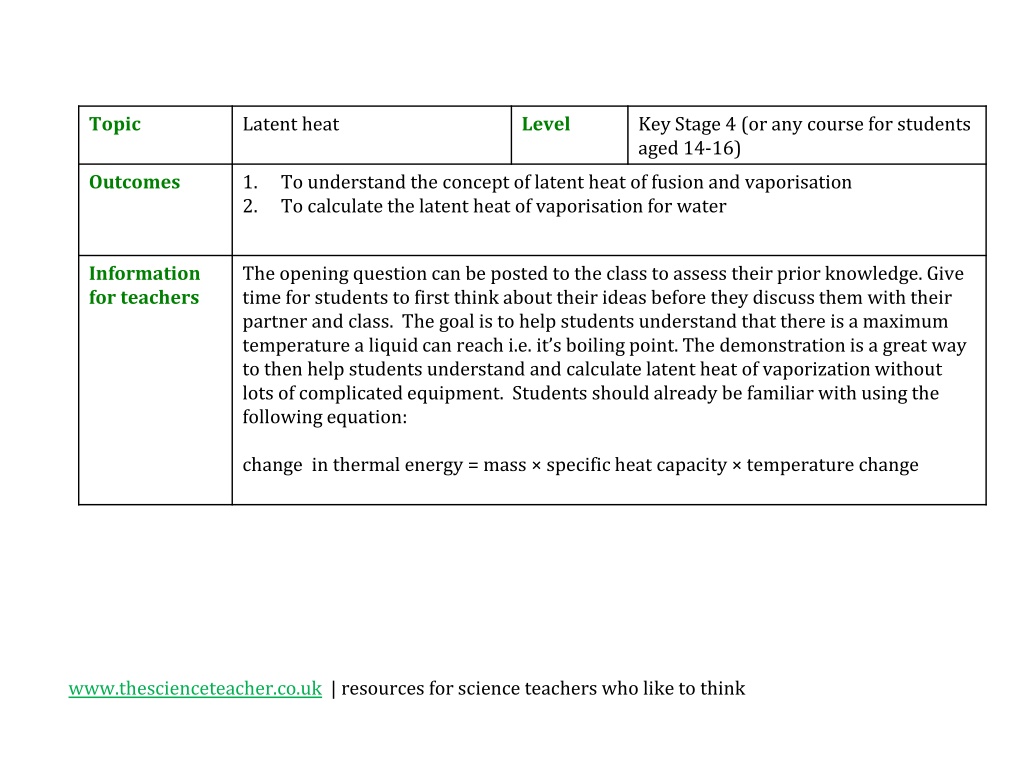
Topic Latent heat Level Key Stage 4 (or any course for students aged 14-16) Outcomes 1. 2. To understand the concept of latent heat of fusion and vaporisation To calculate the latent heat of vaporisation for water Information for teachers The opening question can be posted to the class to assess their prior knowledge. Give time for students to first think about their ideas before they discuss them with their partner and class. The goal is to help students understand that there is a maximum temperature a liquid can reach i.e. it s boiling point. The demonstration is a great way to then help students understand and calculate latent heat of vaporization without lots of complicated equipment. Students should already be familiar with using the following equation: change in thermal energy = mass specific heat capacity temperature change www.thescienceteacher.co.uk | resources for science teachers who like to think
Would you rather be boiled alive in water or olive oil or would it make no difference?!
Once a substance reaches its boiling point its temperature will not increase until all the substance has turned into a vapour. This is because energy is being used to break forces between molecules and not increase the kinetic energy of particles. Olive oil boils at 300 oC and water boils at 100 oC. No matter how long you heat liquid water for, it will never reach a temperature higher than 100 oC.
Method to work out the specific latent heat of vaporisation of water. 1. 2. 3. Fill one beaker with water and one identical beaker with 100 ml of water Place both beakers on a hot plate Measure the initial temperature of the of the full beaker of water and record the temperature again once all the water in the second beaker has evaporated Now use the specific heat capacity of water to calculate the latent heat of vaporisation in joules per kilogram. 4. How much energy does it take to turn liquid water into a vapour? 100 ml Hot plate