India's Best Pharma Products Manufacturers in Chandigarh
Unimarck Pharma India's Best Pharma Products Manufacturers in Chandigarh. We are WHO-GMP, GLP, and ISO certified. 40 years of experience in this industry.
4 views • 7 slides
CPGP Study Guide and How to Crack Exam on Pharmaceutical GMP Professional
Click Here--- \/\/bit.ly\/4bsglWA ---Get complete detail on CPGP exam guide to crack Pharmaceutical GMP Professional. You can collect all information on CPGP tutorial, practice test, books, study material, exam questions, and syllabus. Firm your knowledge on Pharmaceutical GMP Professional and get r
3 views • 35 slides
Pharma Franchise For All Pharma Products All Over India
Unibiotech Formulations offer different types of Pharma products all over India for PCD Pharma Franchise. Unibiotech is WHO-GMP certified Pharma Franchise company. Call us Today @ 917814301804, 919216901651.\n\n\n
2 views • 6 slides
ASQ Pharmaceutical GMP Professional (CPGP) Exam | Boost Your Score
Click Here---> \/\/bit.ly\/4bsglWA <---Get complete detail on CPGP exam guide to crack Pharmaceutical GMP Professional. You can collect all information on CPGP tutorial, practice test, books, study material, exam questions, and syllabus. Firm your knowledge on Pharmaceutical GMP Professional and get
0 views • 20 slides
Pharmaceutical Manufacturing Company in India | Unimarck
Our focus is to provide reliable manufactured products that are well-certified by reputable organizations like WHO and GMP. To provide a diverse range of products like quality tablets, syrups, and capsules, our company is working hard to meet high standards in its manufacturing process.
1 views • 4 slides
Top PCD Pharma Franchise Company in India | WHO GMP Certified
Unibiotech Formulations is WHO GMP Certified Pharma Franchise Company in India. We offer Latest formulations in tablets, capsules, suspensions, syrup, etc. Get more info for franchise Services Contact us at 917814301804
2 views • 5 slides
Understanding GMP Audits in Construction: Navigating Client Expectations
This presentation at the National Association of Construction Auditors' virtual conference focuses on helping clients grasp the key objectives and processes of Guaranteed Maximum Price (GMP) audits. Dave Potak, a seasoned professional, will share insights on managing client expectations, best practi
0 views • 18 slides
Guidelines for Personnel Training and Hygiene in Pharmaceutical Manufacturing
Personnel responsibilities in a manufacturing unit include training, hygiene, and maintaining personal records. Guidelines as per Sch.M of D&C act 1945 outline the supervision, qualifications, and duties required for technical staff, QC lab, and QA personnel. Health, clothing, and sanitation protoco
2 views • 16 slides
Microbiological Quality Control in Pharmaceutical Environment
Microbiological products in pharmaceutical settings are influenced by the quality of materials and the environment. Good Manufacturing Practices (GMP) are essential to minimize contamination risks, with control points focused on the ecology of microorganisms. Sources of contamination include the atm
1 views • 128 slides
Top WHO-GMP-ISO Certified Monopoly Pharma Franchise Company
Top WHO-GMP-ISO Certified Monopoly Pharma Franchise Company in India. Contact Unibiotech Formulations At 917814301804, 919216901651
1 views • 5 slides
ASEAN Guidelines on GMP for Traditional Medicines: Evaluation of Corrective Action and Preventive Action
The ASEAN Guidelines on GMP for Traditional Medicines discuss the importance of Corrective Action and Preventive Action (CAPA) for maintaining quality in health supplements. CAPA involves identifying nonconformities, implementing solutions, and preventing future occurrences through continuous improv
1 views • 47 slides
Managing Microbiological Quality in Pharmaceutical Environments
The quality of microbiological products is influenced by the pharmaceutical environment materials. Good Manufacturing Practices (GMP) play a crucial role in minimizing contamination risks. Various sources of contamination include the atmosphere, water, persons, raw materials, packaging, buildings, a
0 views • 128 slides
WHO-GMP, GLP, ISO Certified Pharma Manufacturing Company
Unimarck Pharma is WHO-GMP, GLP, ISO Certified Pharma Manufacturing Company Since 1984. Contact us today at 91-172-2244500.
1 views • 5 slides
Understanding Good Laboratory Practices (GLP) in Pharmaceuticals
Good Laboratory Practices (GLP) is a quality system ensuring non-clinical health and environmental safety studies are conducted accurately and reliably. GLP promotes the validity of test data for determining safety of chemicals, pharmaceuticals, food, and cosmetics. This article explores the definit
0 views • 40 slides
ASEAN Guidelines on GMP for Traditional Medicines/Health Supplements - Preparation for Inspection
This content provides guidelines on preparing for a Good Manufacturing Practice (GMP) inspection for traditional medicines and health supplements in ASEAN countries. It covers activities such as planning inspections, forming inspection teams, reviewing documentation, preparing inspection plans, hold
0 views • 19 slides
Glycomacropeptide Extraction and Casein Curd Formation from Skim Milk
This detailed study explores the extraction of glycomacropeptide (GMP) and securing casein curd from skim milk. It covers the composition of milk, the classification of milk proteins, the role of rennet in curd formation, and the history and preparation of rennet. Additionally, it delves into the us
0 views • 34 slides
ASEAN Guidelines on GMP for Traditional Medicines - Preparation of GMP Report
The ASEAN Guidelines on GMP for Traditional Medicines provide detailed instructions on preparing GMP reports, including post-inspection activities, deficiency classification, examples of deficiencies, and inspection report format. Deficiencies are categorized as Critical, Major, or Minor, with speci
0 views • 20 slides
ASEAN Guidelines on GMP for Traditional Medicines - Philosophy of Inspection
The ASEAN Guidelines on GMP for Traditional Medicines highlight the Philosophy of Inspection for ensuring quality and safety in traditional medicines and health supplements. The document covers legal terms, audit trail requirements, electronic signature control, and familiar auditor requirements. It
0 views • 88 slides
Enhancing Feed Safety Through GMP+ International Certification
Explore the world of GMP+ International certification for feed safety, providing value to former foodstuffs. Learn how food companies can ensure safe feed practices, with a focus on compliance, incident management, and traceability. Discover the chain approach in various industries, from cultivation
0 views • 12 slides
Analysis of Boiling Target Study at Kent State University
Boiling Target Study conducted at Kent State University focused on analyzing the impact of beam current on target density fluctuations, termed as Boiling. The study involved applying various cuts, calculating live time, and extracting charge yield data to determine the presence of target boiling. Su
0 views • 12 slides
ASEAN Guidelines on GMP for Traditional Medicines/Health Supplements: Conducting GMP Inspection
The ASEAN Guidelines provide detailed procedures for conducting GMP inspections to ensure objectivity and appropriateness. The inspection processes include opening meetings, facility inspections, documentation review, inspector meetings, and exit meetings. During the opening meeting, the inspection
0 views • 30 slides
Pharma Franchise Company For Start Your Pharma Business
Pharma Franchise Company For Start Your Pharma Business. Unibiotech Formulations, a WHO-GMP certified PCD Pharma company in India. Call 7814301804, 9216901651 For Full Details.
3 views • 4 slides
ASEAN Guidelines on GMP for Traditional Medicines - Classification of GMP Non-Conformance
Classification of GMP non-conformance is crucial for conducting inspections and preparing reports. It helps companies take necessary actions and affects the inspection rating. The guidelines outline critical, major, minor, and other deficiencies in traditional medicines and health supplements, empha
0 views • 26 slides
Importance of Personnel Hygiene in Dairy Plant Management
In a dairy plant, maintaining good manufacturing practices (GMP) is crucial for ensuring quality and safety of products. This includes emphasizing personnel hygiene, cleanliness, and preventing contamination. Employees must adhere to strict personal hygiene practices, such as proper grooming and wea
0 views • 15 slides
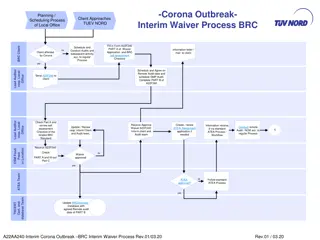
Interim Waiver Process for BRC Audits During Corona Outbreak
This content outlines the interim waiver process and scheduling procedures for local office client audits during the Corona outbreak. It includes steps for completing waiver applications, conducting remote audits, and handling certification extensions. The document also provides guidelines for remot
0 views • 11 slides
RAC Exam I Test Questions
The RAC Exam I Test Questions cover various aspects of regulatory affairs and compliance in the pharmaceutical industry. The questions touch on topics such as GMP, FDA regulations, product approvals, and legal requirements. Test your knowledge with these 100 questions to enhance your understanding o
0 views • 101 slides
ASEAN Guidelines on GMP for Traditional Medicines - Preparation for Inspection
This content outlines the preparation activities for a Good Manufacturing Practice (GMP) inspection for Traditional Medicines/Health Supplements as specified by the ASEAN Guidelines. It covers the objectives, processes, inspection team formation, documentation review, and other key aspects involved
0 views • 19 slides