Periodic Law and the Periodic Table
The Periodic Law dictates the predictable patterns in physical and chemical properties of elements when arranged by atomic number. The discovery and impact of the Periodic Law, along with the properties it affects like atomic radius, ionization energy, electronegativity, and more, are essential in u
8 views • 12 slides
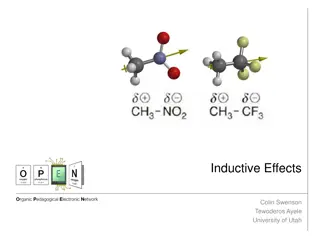
Understanding Inductive Effects in Organic Chemistry
Inductive effects play a crucial role in the reactivity and properties of organic molecules. This phenomenon involves the withdrawal or donation of electron density due to differences in electronegativity, impacting acidity, stability, and interactions with other molecules. Explore how inductive eff
0 views • 7 slides
Understanding Polar Bonds and Molecules in Chemistry
Learn about polar and nonpolar covalent bonds, the classification of bonds based on electronegativity differences, and how to identify polar molecules through unequal sharing of electrons. Practice determining bond types and grasp the concept of partial charges in polar bonds.
0 views • 18 slides
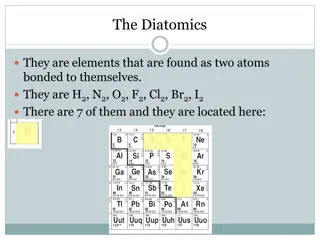
Exploring Periodic Trends and Diatomic Elements
Dive into the world of periodic trends, atomic size variations, ionization energy, electronegativity, and diatomic elements like H2, N2, O2, F2, Cl2, Br2, and I2. Discover how atomic structure influences these properties and learn about the fascinating characteristics of cations, anions, and shieldi
0 views • 12 slides
General Overview of p-Block Elements in Chemistry
p-block elements in the periodic table include metals, non-metals, metalloids, and noble gases from Group IIIA to Group VIIA. This group of elements has unique electronic configurations, properties, and characteristics, making them essential in understanding chemical behavior. The content discusses
0 views • 47 slides
Understanding Molecular Polarity and Electronegativity in Chemistry
Explore the concept of molecular polarity, electronegativity, and bonding in chemistry through engaging visuals and discussions. Learn how the unequal sharing of electrons between atoms leads to polarity in molecules and discover the role of electronegativity in attracting shared electrons. Delve in
0 views • 12 slides
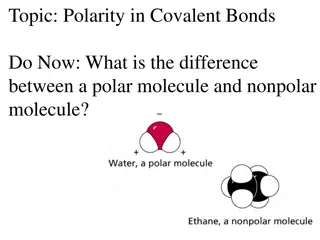
Understanding Polarity in Covalent Bonds
The difference between a polar molecule and a nonpolar molecule lies in the distribution of electrons. A polar molecule has an asymmetric electron distribution due to a significant difference in electronegativity, while a nonpolar molecule has a symmetric electron distribution. You can predict polar
0 views • 15 slides
Understanding Polarity, Electronegativity, and Chemical Bonds
Delve into the concepts of polarity, electronegativity, and different types of chemical bonds by exploring the tug-of-war analogy and examples of polar, nonpolar, ionic, and covalent bonds. Learn how electronegativity values determine the nature of bonds and the sharing of electrons in molecules.
0 views • 17 slides
Transition Metal Catalyzed Trifluoromethylation and Its Importance in Pharmaceutical and Material Science
Transition metal catalyzed trifluoromethylation is a valuable method for introducing trifluoromethyl groups into unactivated alkenes, with significant impact in pharmaceutical, agrochemistry, and material science. The incorporation of CF3 is essential due to its enhancement of metabolic stability, p
0 views • 24 slides
Understanding Periodic Trends in Atomic Characteristics
Periodic trends in atomic characteristics such as atomic radius, ionization energy, ion size, and electronegativity are essential in understanding the behavior of elements. These trends are influenced by factors like the energy levels and the number of protons and electrons in an atom. The atomic ra
0 views • 23 slides
Understanding the Evolution and Properties of the Periodic Table
Explore the history and organization of the periodic table, including changes over time, classifications of elements as metals, non-metals, and metalloids, and the formation of ions. Learn about key vocabulary, valence electrons, ionic trends, and various elemental properties like electronegativity
0 views • 42 slides
Factors Affecting Acid Strength: Atoms, Size, Hybridization, and Electronegativity
The strength of acids is influenced by various factors such as the atom on which the negative charge of the acid's conjugate base rests, atom size, hybridization, and electronegativity. The stability of negative charges on atoms, atom size allowing charge delocalization, preferred orbital types for
0 views • 23 slides
Exploring the Nitrogen Family: Properties, Characteristics, and More
Delve into the Nitrogen Family, consisting of two nonmetals, metalloids, and a metal. Learn about their unique properties, electron configurations, atomic symbols, and ionic radii. Discover how ionization enthalpy and electronegativity vary within the group, shedding light on the behavior of these e
0 views • 11 slides
Lemon-Powered Car Experiment: Creating Electricity from Citrus Cells
The Lemon-Powered Car experiment involves determining the potency of reducing agents, creating chemical cells to generate electricity, utilizing capacitors to store and release energy, and building a car powered by a chemical reaction. Key concepts covered include redox reactions, batteries, capacit
0 views • 21 slides
Understanding the Periodic Table: Elements, Trends, and Atomic Radius
The periodic table organizes elements by properties, with Mendeleev's contributions and predictions. Elements are grouped into periods and groups, displaying trends in ionization energy, electronegativity, and atomic size. Learn about the atomic radius, how it varies across periods and groups, influ
0 views • 20 slides
Understanding Electrochemistry: A Comprehensive Overview
Dive into the world of electrochemistry with this detailed review covering topics such as oxidation, reduction, half-reactions, and the significance of oxidation numbers in balancing redox equations. Explore key concepts like OIL RIG (Oxidation Is Loss, Reduction Is Gain) and learn how to apply elec
0 views • 17 slides